-
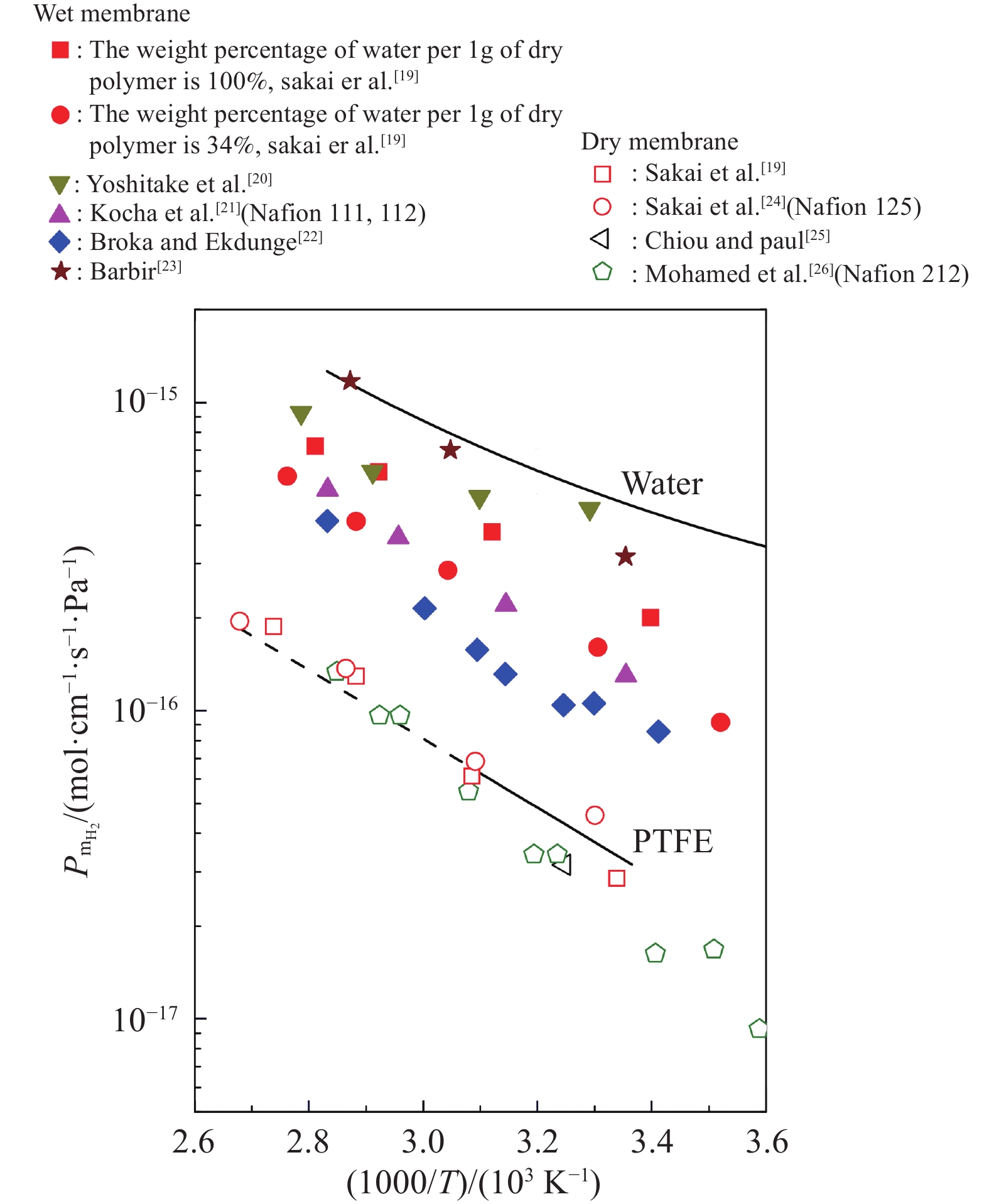
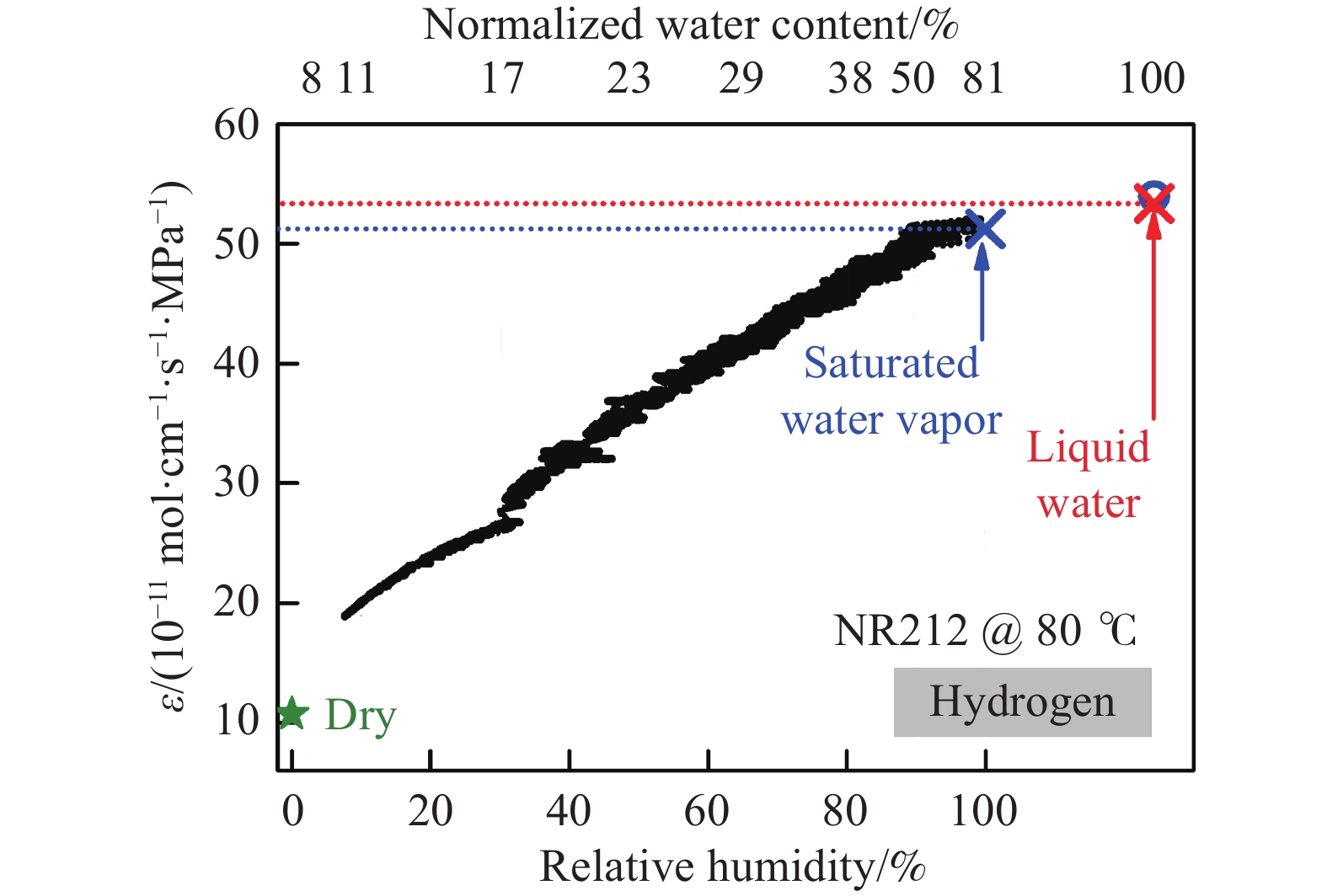
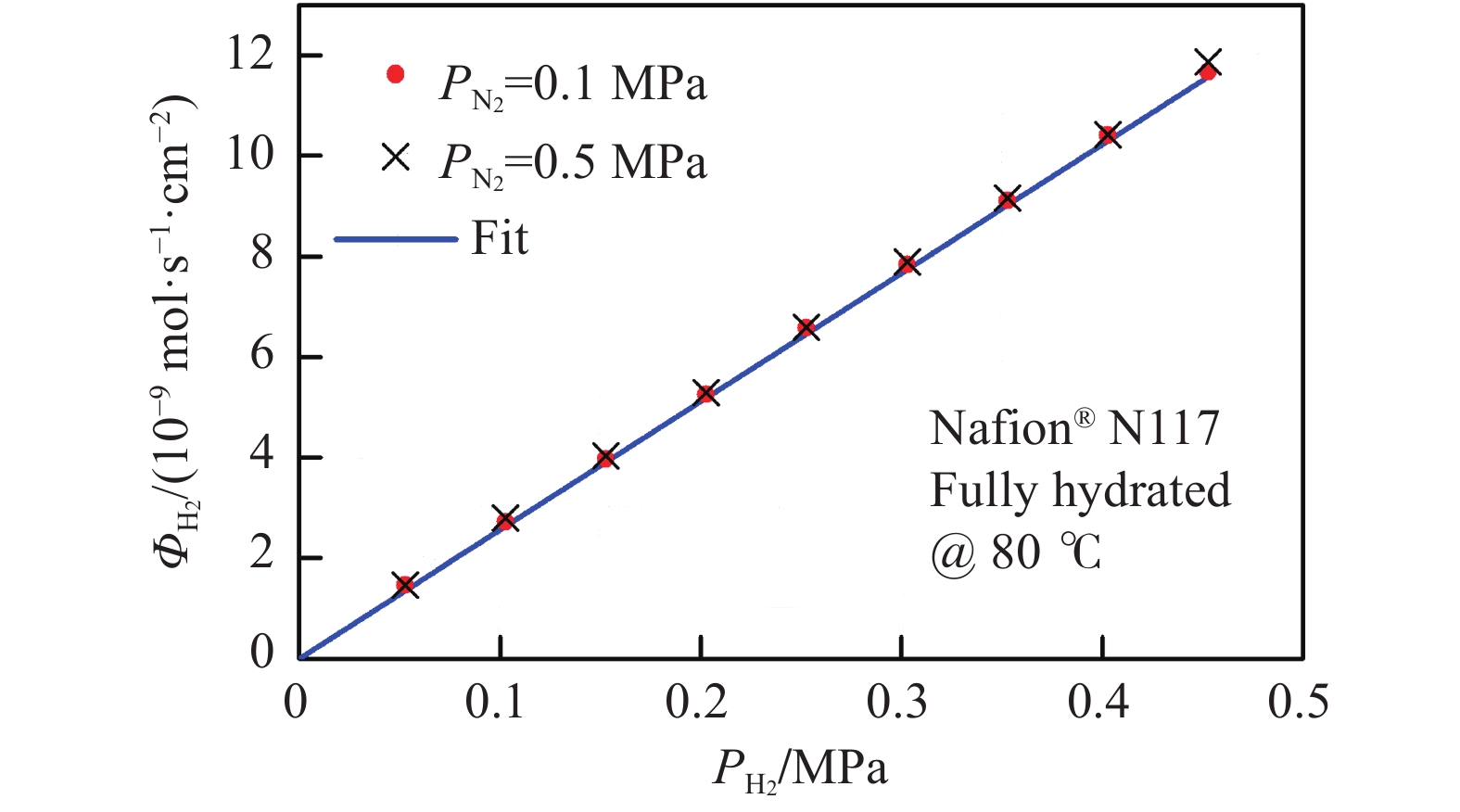
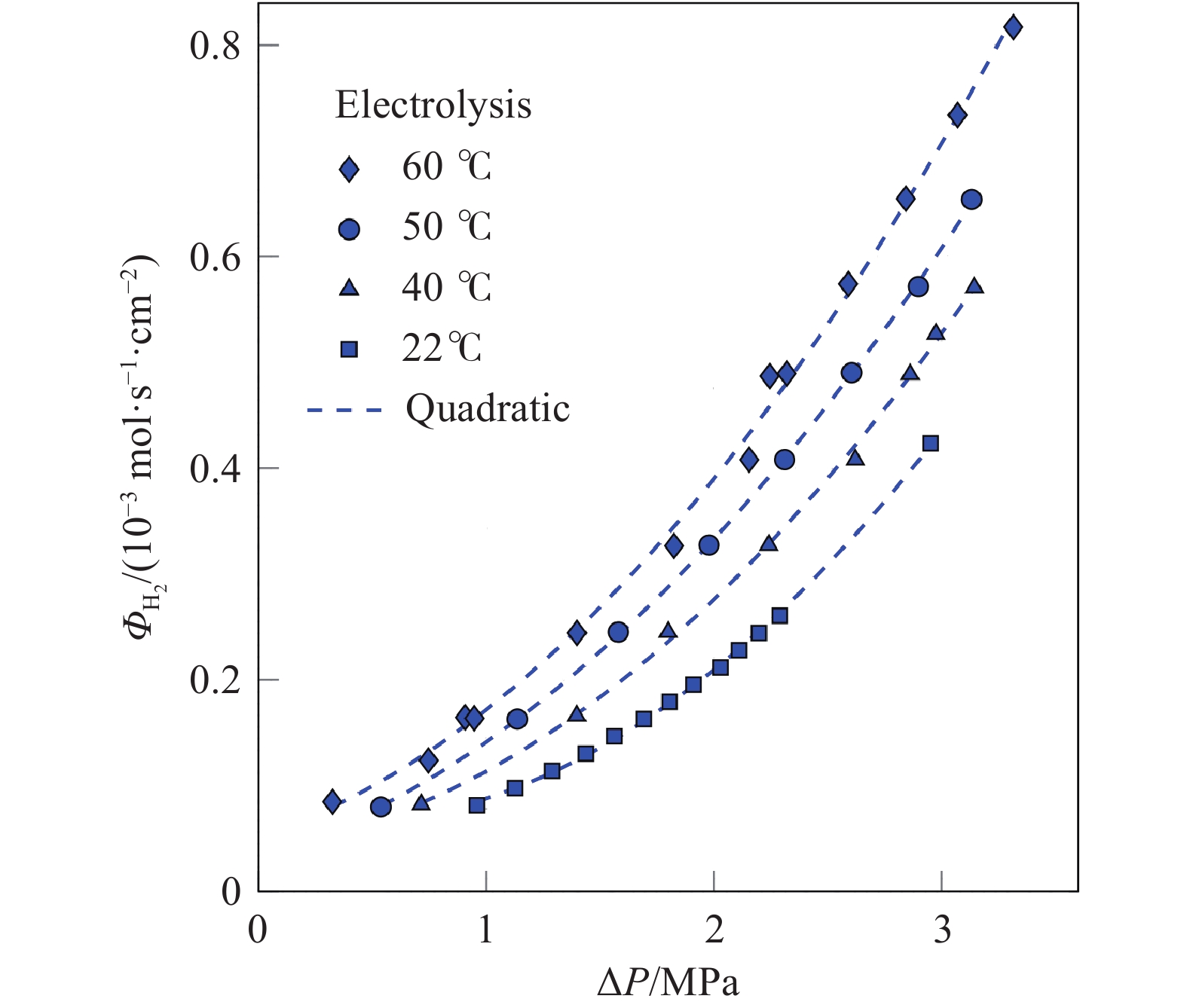
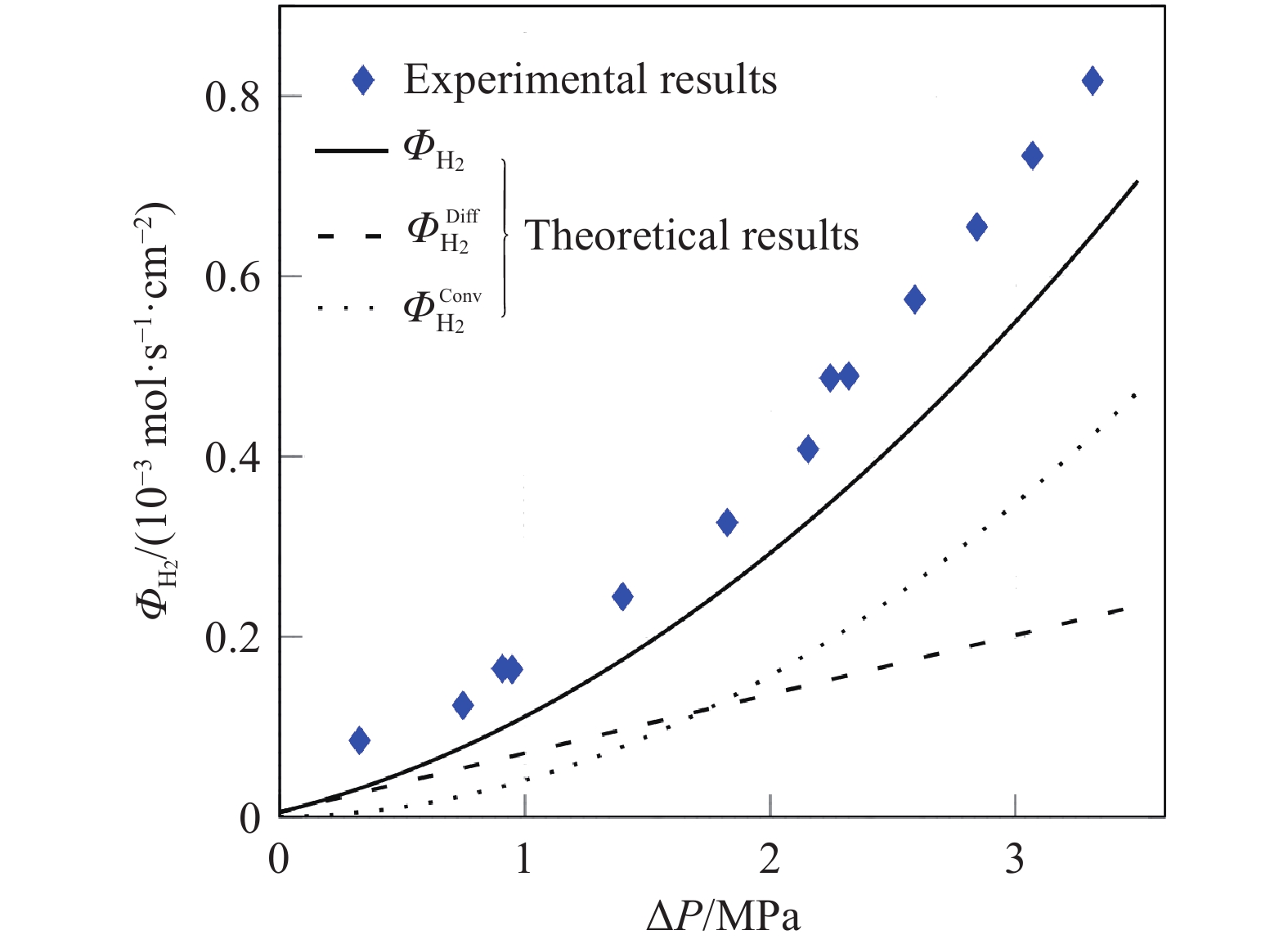
摘要: 由于膜的吸水特性,高壓質子交換膜(PEM)制氫(尤其是差壓式,氫側高壓/氧側常壓)存在氫氣滲透問題,影響電解堆的運行安全與效率。基于菲克定律描述的滲透通量與滲透率、分壓差的關系,綜述了溫度/壓力、膜水合程度、氫氣分壓差對氫氣滲透的影響規律。在常規運行壓力范圍(3.5 MPa)內,擴散系數與溶解度主要受溫度影響,溫度升高則滲透率增大;氫氣滲透率隨膜水合程度的增加而增大;氫氣分壓差對滲透的影響表現出線性(滲透池環境)與非線性(電解制氫環境)兩種關系,非線性可能源于膜透水性提升與水通道結構改變引起的對流滲透。考慮到電解制氫實際工況存在電流,綜述了電流密度對氫滲透的影響,氫氣滲透率隨運行電流密度的升高而增大,氫過飽和是可能的影響機理,高電流密度下氫過飽和度升高,導致氫氣通過膜的滲透增加。Abstract: Hydrogen has the advantages of high energy density, no pollution, and long-term storage. As an important medium for the transformation of energy interconnection, it helps to promote the clean and efficient use of traditional fossil energy, support the large-scale development of renewable energy, and achieve large-scale deep decarbonization. With the excellent responsiveness to intermittent and fluctuating power supplies, proton exchange membrane (PEM) water electrolysis has been a research hotspot in the field of hydrogen production with renewable energy and will be one of the main technical routes for effective hydrogen supply in the future. The high-pressure operation of PEM electrolyzers has been successfully realized and commercialized, considering PEM’s outstanding mechanical strength and gas separation properties. However, due to the water-absorbing properties of the membrane, an important problem in high-pressure PEM water electrolysis (especially under differential pressure conditions and high pressure in the cathode compartment/atmospheric pressure in the anode compartment) is the permeation of hydrogen through the membrane, which affects safety and efficiency. In this article, the research progress of hydrogen permeation in PEM electrolysis was reviewed. First, the theory of permeation was introduced. Second, considering the relationship among the permeation flux, permeability, and partial pressure difference described by Fick’s law, the effects of temperature/pressure, hydration degree of the membrane, and partial pressure difference on hydrogen permeation were reviewed. In the normal operating pressure range (<3.5 MPa) for hydrogen production through PEM electrolysis, the diffusion coefficient and solubility are mainly affected by temperature, and the permeability increases with increasing temperature. The permeability of hydrogen in water is approximately 5–10 times that of a dry film, and the permeability increases with increases in the relative humidity of the membrane. The influence of partial pressure difference in hydrogen permeation shows a linear dependence in permeation cell and quadratic dependence in real electrolysis. The quadratic dependence may be attributed to the convective permeation caused by the increase in membrane water permeability and changes in the water channel. Third, considering the current in real operating conditions of electrolysis, the effect of current density on hydrogen permeation was reviewed. The permeability increases with the increase in current density, which may be attributed to the increase in hydrogen supersaturation in the cathode. At a high current density, the hydrogen concentration within the ionomer at the cathode catalyst particles become higher, and the high concentration gradient causes hydrogen to diffuse from cathode to anode.
-
Key words:
- PEM water electrolysis /
- differential pressure /
- hydrogen permeation /
- permeability /
- current density
-
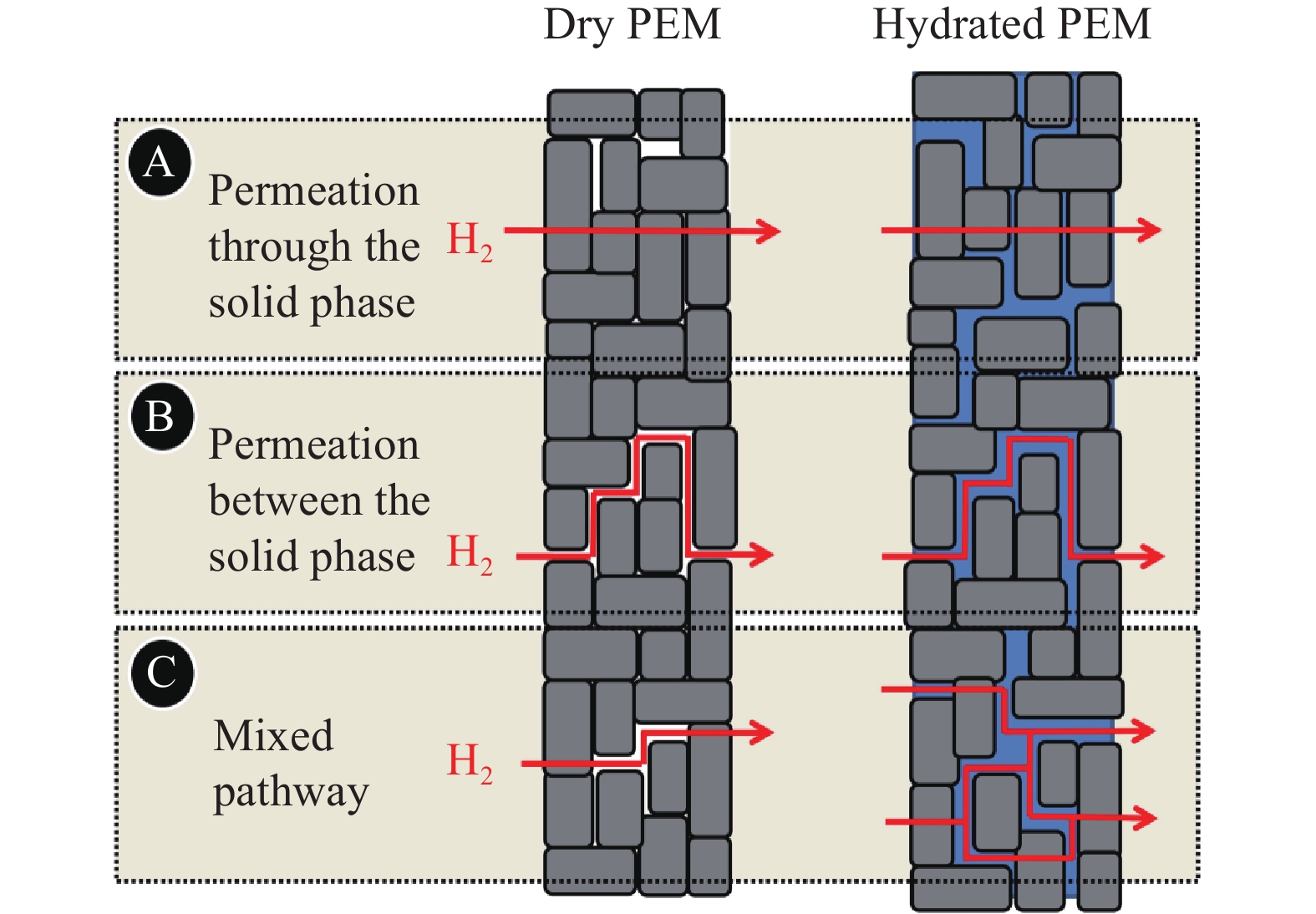
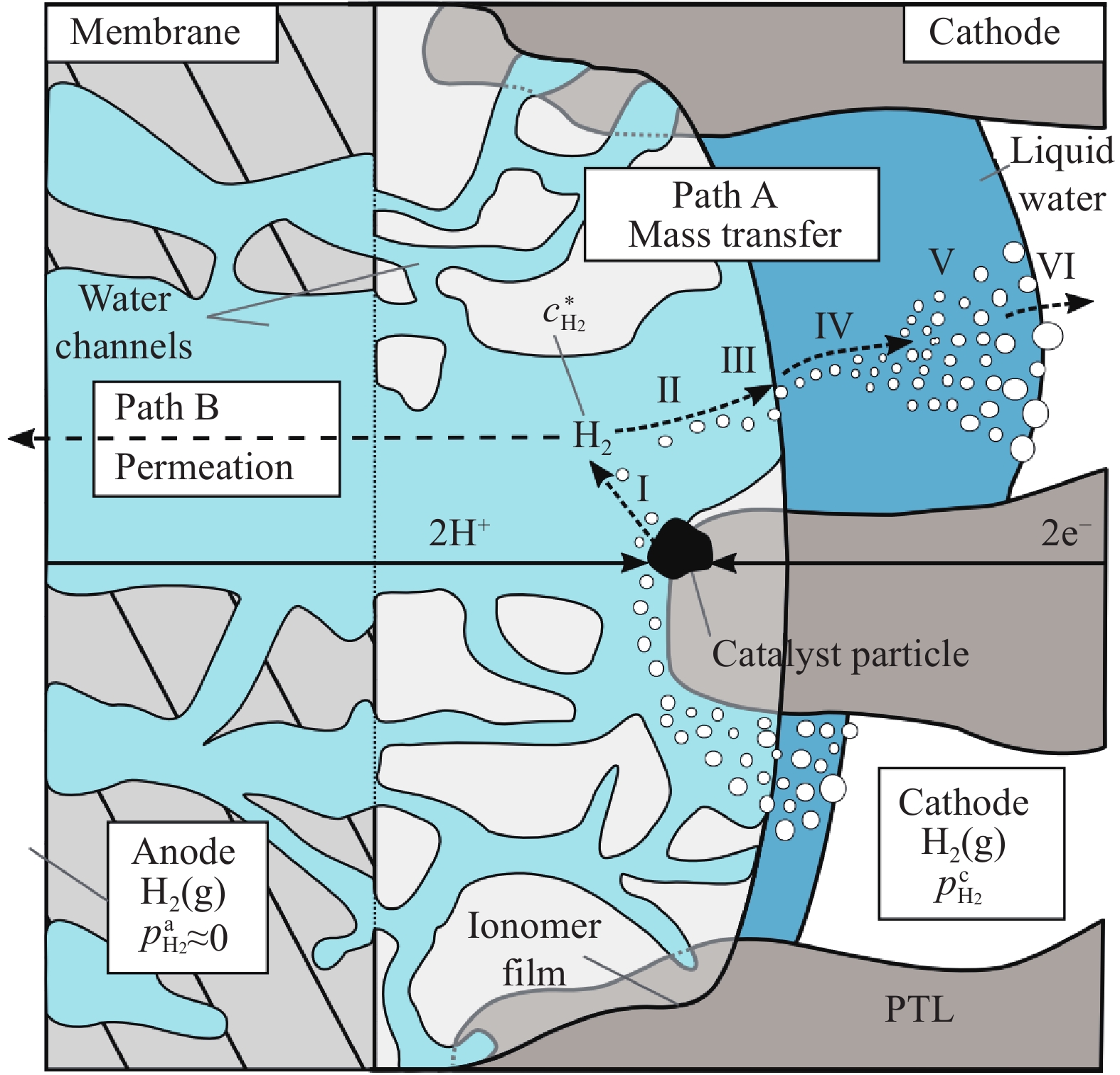
圖 4 氫氣以不同路徑滲透通過PEM的示意圖(灰色區域代表固相, 藍色區域代表水相, 白色代表孔洞; 左側為干膜, 右側為濕膜)[14]
Figure 4. Descriptive sketch of the pathways for gas permeation through a segment of PEM exemplified for hydrogen molecules (The solid polymeric phase is depicted as the gray area, water as the blue area, and pores filled with gas as the white area; left: dry PEM; right: hydrated PEM)[14]
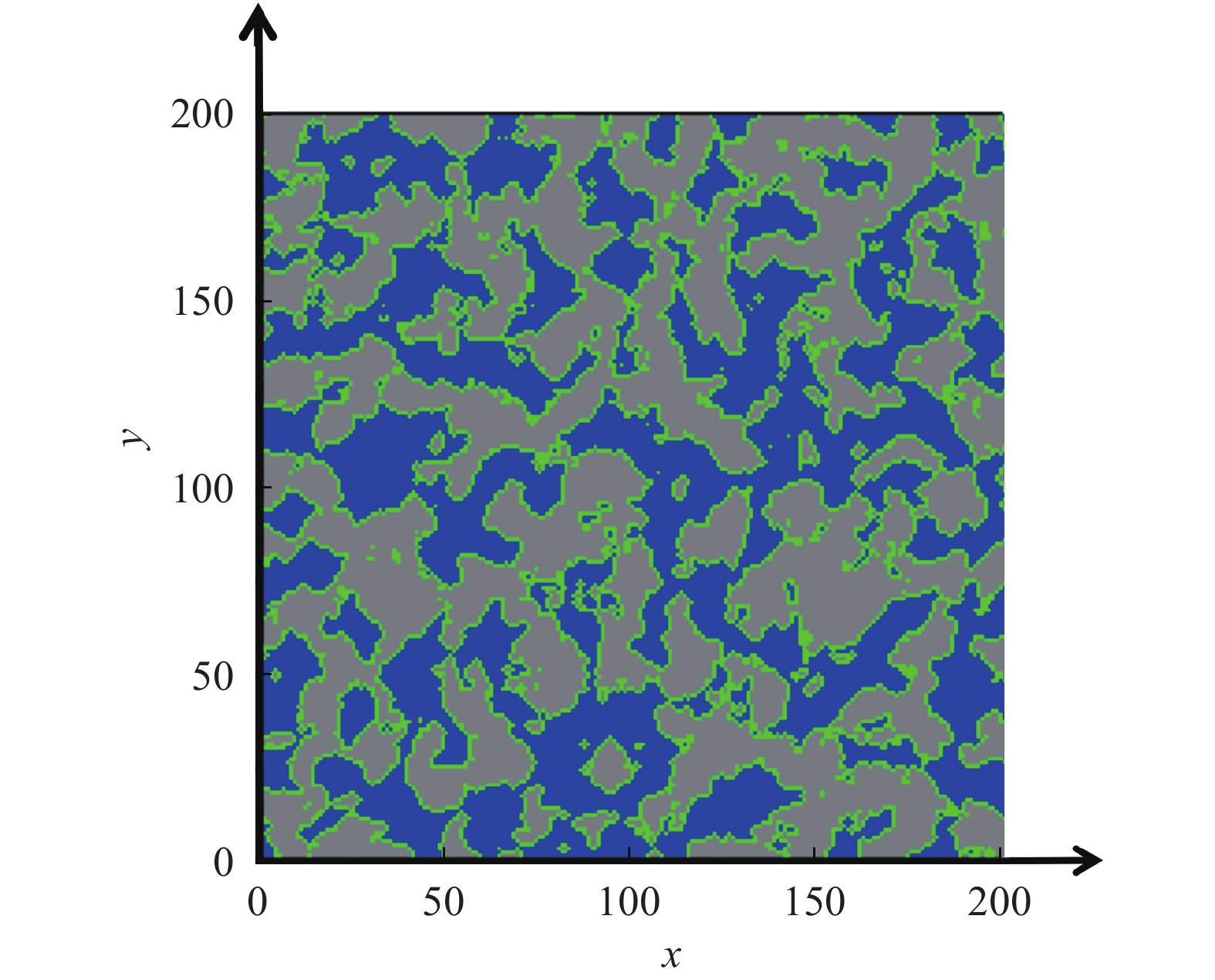
圖 5 Nafion膜三維結構模型的截面圖,圖中橫縱坐標表示200個網格單位,每個網格單位對應邊長為0.21 nm的立方體(灰色區域: 固相, 藍色區域: 水相, 綠色區域: 中間相)[27]
Figure 5. Two-dimensional cross section of the modeled three-dimensional cubic structure of Nafion with an edge length of 200 segments. One segment of the mesh corresponds to a cube with 0.21 nm edge length. (Gray area: solid phase; blue area: aqueous phase; green area: intermediate phase)[27]
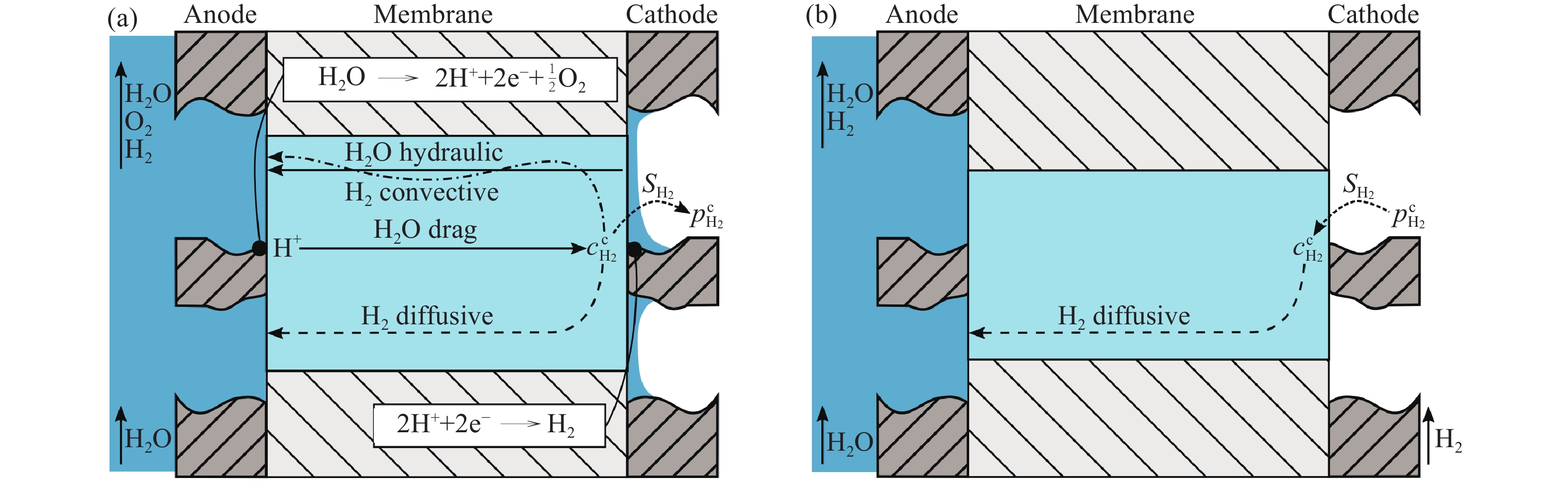
圖 9 測量條件示意圖. (a) 水電解條件下測量; (b) 滲透池條件下測量(未施加電流)[28]
Figure 9. Schematic measurement conditions for (a) measurement during electrolysis and (b) measurement in a permeation cell without applying current[28]
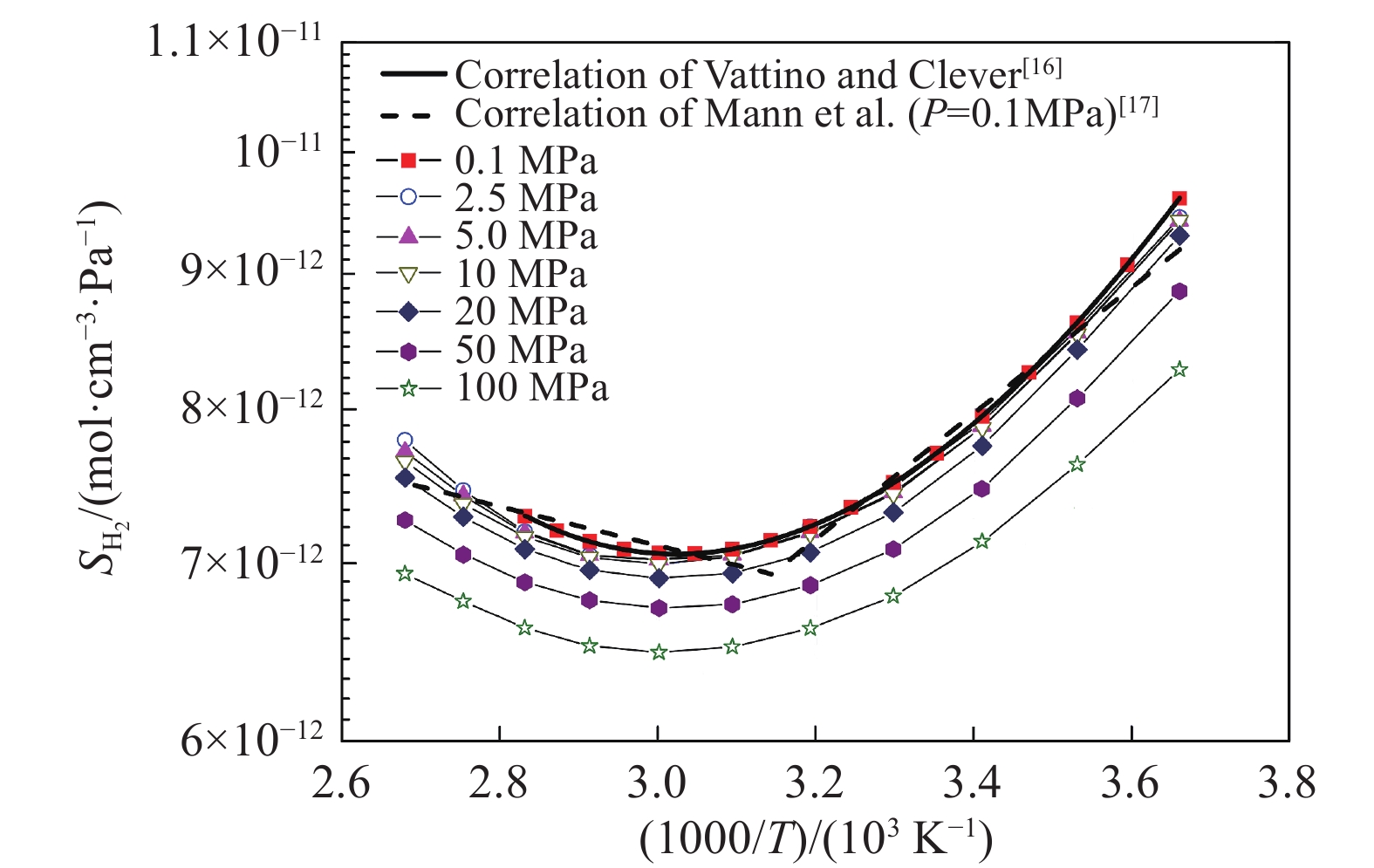
Note: $ c_{{{\text{H}}_{\text{2}}}}^{\text{c}} $ is the dissolved hydrogen concentration in the cathode, mol·m-3; $ {S_{{{\text{H}}_{\text{2}}}}} $ is the solubility of hydrogen in water, mol·m-3·Pa-1; $ p_{{{\text{H}}_{\text{2}}}}^{\text{c}} $ is the hydrogen pressure in the cathode, Pa
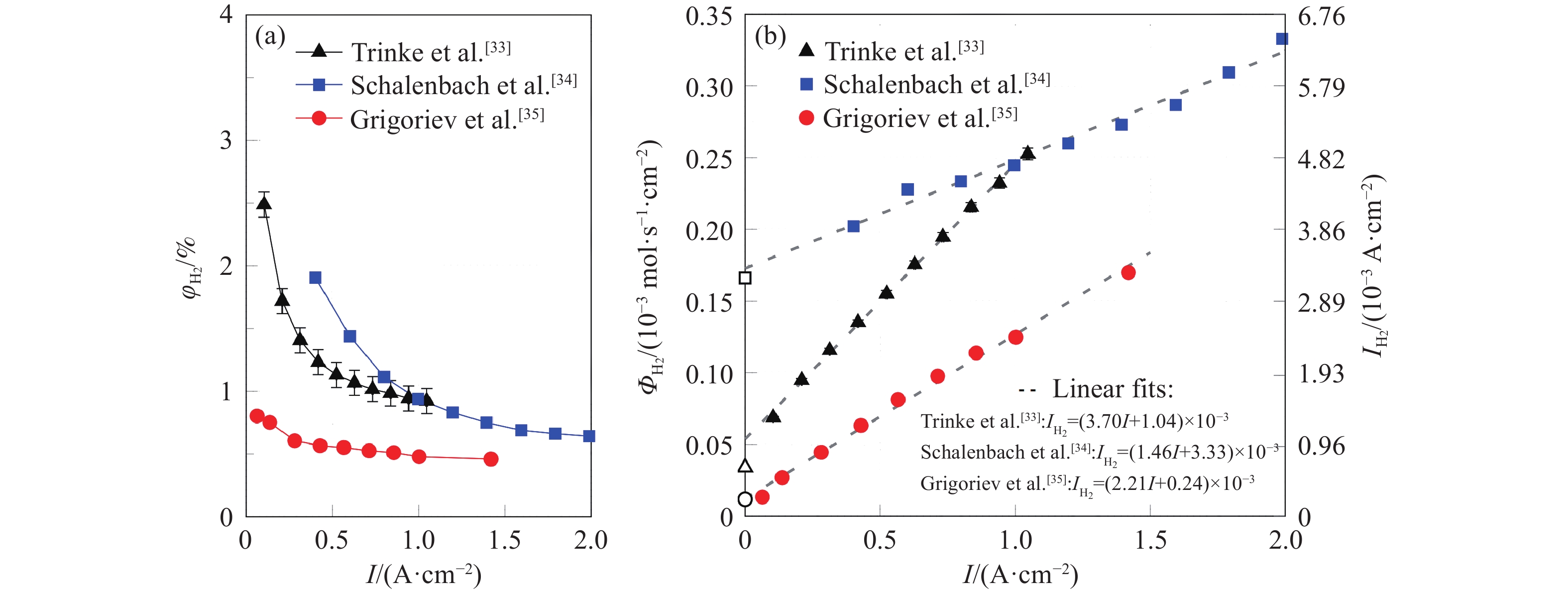
久色视频圖 11 陰極催化層離聚物中氫過飽和示意圖[33]
Figure 11. Sketch of the hydrogen supersaturation within the ionomer film of the cathode[33]
Note:$ c_{{{\text{H}}_{\text{2}}}}^ * $is the dissolved hydrogen concentration within the ionomer, mol·m−3; $ p_{{{\text{H}}_{\text{2}}}}^{\text{a}} $ is the hydrogen pressure in the anode, Pa; $ p_{{{\text{H}}_{\text{2}}}}^{\text{c}} $is the hydrogen pressure in the cathode, Pa; PTL is Porous transport layer
-
參考文獻
[1] Grigoriev S A, Fateev V N, Bessarabov D G, et al. Current status, research trends, and challenges in water electrolysis sience and technology. Int J Hydrog Energy, 2020, 45(49): 26036 doi: 10.1016/j.ijhydene.2020.03.109 [2] Liu S M, Deng Z F, Xu G Z, et al. Commercialization and future development of the solid oxide fuel cell (SOFC) in Europe. Chin J Eng, 2020, 42(3): 278劉少名, 鄧占鋒, 徐桂芝, 等. 歐洲固體氧化物燃料電池(SOFC)產業化現狀. 工程科學學報, 2020, 42(3):278 [3] Kumar S S, Himabindu V. Hydrogen production by PEM water electrolysis-A review. Mater Sci Energy Technol, 2019, 2(3): 442 [4] Ayers K. High efficiency PEM water electrolysis: Enabled by advanced catalysts, membranes, and processes. Curr Opin Chem Eng, 2021, 33: 100719 doi: 10.1016/j.coche.2021.100719 [5] Koponen J, Kosonen A, Ruuskanen V, et al. Control and energy efficiency of PEM water electrolyzers in renewable energy systems. Int J Hydrog Energy, 2017, 42(50): 29648 doi: 10.1016/j.ijhydene.2017.10.056 [6] Suermann M, P?tru A, Schmidt T J, et al. High pressure polymer electrolyte water electrolysis: Test bench development and electrochemical analysis. Int J Hydrog Energ, 2017, 42(17): 12076 doi: 10.1016/j.ijhydene.2017.01.224 [7] Lee B, Heo J, Kim S, et al. Economic feasibility studies of high pressure PEM water electrolysis for distributed H2 refueling stations. Energy Convers Manag, 2018, 162: 139 doi: 10.1016/j.enconman.2018.02.041 [8] Sartory M, Walln?fer-Ogris E, Salman P, et al. Theoretical and experimental analysis of an asymmetric high pressure PEM water electrolyser up to 155 bar. Int J Hydrog Energy, 2017, 42(52): 30493 doi: 10.1016/j.ijhydene.2017.10.112 [9] Papakonstantinou G, Sundmacher K. H2 permeation through N117 and its consumption by IrOx in PEM water electrolyzers. Electrochem Commun, 2019, 108: 106578 doi: 10.1016/j.elecom.2019.106578 [10] Afshari E, Khodabakhsh S, Jahantigh N, et al. Performance assessment of gas crossover phenomenon and water transport mechanism in high pressure PEM electrolyzer. Int J Hydrog Energy, 2021, 46(19): 11029 doi: 10.1016/j.ijhydene.2020.10.180 [11] Siracusano S, Trocino S, Briguglio N, et al. Analysis of performance degradation during steady-state and load-thermal cycles of proton exchange membrane water electrolysis cells. J Power Sources, 2020, 468: 228390 doi: 10.1016/j.jpowsour.2020.228390 [12] Khatib F N, Wilberforce T, Ijaodola O, et al. Material degradation of components in polymer electrolyte membrane (PEM) electrolytic cell and mitigation mechanisms: A review. Renew Sustain Energy Rev, 2019, 111: 1 doi: 10.1016/j.rser.2019.05.007 [13] Frensch S H, Fouda-Onana F, Serre G, et al. Influence of the operation mode on PEM water electrolysis degradation. Int J Hydrog Energy, 2019, 44(57): 29889 doi: 10.1016/j.ijhydene.2019.09.169 [14] Schalenbach M, Hoefner T, Paciok P, et al. Gas permeation through nafion. part 1: Measurements. J Phys Chem C, 2015, 119(45): 25145 [15] Ito H, Maeda T, Nakano A, et al. Properties of Nafion membranes under PEM water electrolysis conditions. Int J Hydrog Energy, 2011, 36(17): 10527 doi: 10.1016/j.ijhydene.2011.05.127 [16] Battino R, Clever H L. The solubility of gases in liquids. Chem?Rev, 1966, 66(4): 395 [17] Mann R F, Amphlett J C, Peppley B A, et al. Henry's Law and the solubilities of reactant gases in the modelling of PEM fuel cells. J Power Sources, 2006, 161(2): 768 doi: 10.1016/j.jpowsour.2006.05.054 [18] Wise D L, Houghton G. The diffusion coefficients of ten slightly soluble gases in water at 10?60 ℃. Chem Eng Sci, 1966, 21(11): 999 doi: 10.1016/0009-2509(66)85096-0 [19] Sakai T, Takenaka H, Wakabayashi N, et al. Gas permeation properties of solid polymer electrolyte (SPE) membranes. J Electrochem Soc, 1985, 132(6): 1328 doi: 10.1149/1.2114111 [20] Yoshitake M, Tamura M, Yoshida N, et al. Studies of perfluorinated ion exchange membranes for polymer electrolyte fuel cells. Denki Kagaku, 1996, 64(6): 727 doi: 10.5796/kogyobutsurikagaku.64.727 [21] Kocha S S, Yang J D, Yi J S. Characterization of gas crossover and its implications in PEM fuel cells. Aiche J, 2006, 52(5): 1916 doi: 10.1002/aic.10780 [22] Broka K, Ekdunge P. Oxygen and hydrogen permeation properties and water uptake of Nafion? 117 membrane and recast film for PEM fuel cell. J Appl Electrochem, 1997, 27(2): 117 doi: 10.1023/A:1018469520562 [23] Barbir F. PEM electrolysis for production of hydrogen from renewable energy sources. Sol Energy, 2005, 78(5): 661 doi: 10.1016/j.solener.2004.09.003 [24] Sakai T, Takenaka H, Torikai E. Gas diffusion in the dried and hydrated nafions. J Electrochem Soc, 1986, 133(1): 88 doi: 10.1149/1.2108551 [25] Chiou J S, Paul D R. Gas permeation in a dry Nafion membrane. Ind Eng Chem Res, 1988, 27(11): 2161 doi: 10.1021/ie00083a034 [26] Mohamed H F M, Ito K, Kobayashi Y, et al. Free volume and permeabilities of O2 and H2 in Nafion membranes for polymer electrolyte fuel cells. Polymer, 2008, 49(13-14): 3091 doi: 10.1016/j.polymer.2008.05.003 [27] Schalenbach M, Hoeh M A, Gostick J T, et al. Gas?permeation through?nafion. part 2: Resistor network model. J Phys Chem C, 2015, 119(45): 25156 [28] Trinke P, Bensmann B, Reichstein S, et al. Hydrogen permeation in PEM electrolyzer cells operated at asymmetric pressure conditions. J Electrochem Soc, 2016, 163(11): F3164 doi: 10.1149/2.0221611jes [29] Duan Q J, Wang H P, Benziger J. Transport of liquid water through Nafion membranes. J Membr Sci, 2012, 392-393: 88 doi: 10.1016/j.memsci.2011.12.004 [30] Sellin R C, Mozet K, Ménage A, et al. Measuring electro-osmotic drag coefficients in PFSA membranes without any diffusion assumption. Int J Hydrog Energy, 2019, 44(45): 24905 doi: 10.1016/j.ijhydene.2019.07.076 [31] Wakita H, Kawabata N, Kani Y. Measurement of water permeation through membranes from extremely high hydraulic pressure to atmospheric pressure. Int J Hydrog Energy, 2019, 44(59): 31257 doi: 10.1016/j.ijhydene.2019.10.037 [32] Shin H S, Oh B S. Water transport according to temperature and current in PEM water electrolyzer. Int J Hydrog Energy, 2020, 45(1): 56 doi: 10.1016/j.ijhydene.2019.10.209 [33] Trinke P, Bensmann B, Hanke-Rauschenbach R. Current density effect on hydrogen permeation in PEM water electrolyzers. Int J Hydrog Energy, 2017, 42(21): 14355 doi: 10.1016/j.ijhydene.2017.03.231 [34] Schalenbach M, Carmo M, Fritz D L, et al. Pressurized PEM water electrolysis: Efficiency and gas crossover. Int J Hydrog Energy, 2013, 38(35): 14921 doi: 10.1016/j.ijhydene.2013.09.013 [35] Grigoriev S A, Millet P, Korobtsev S V, et al. Hydrogen safety aspects related to high-pressure polymer electrolyte membrane water electrolysis. Int J Hydrog Energy, 2009, 34(14): 5986 doi: 10.1016/j.ijhydene.2009.01.047 [36] Ito H, Miyazaki N, Ishida M, et al. Cross-permeation and consumption of hydrogen during proton exchange membrane electrolysis. Int J Hydrog Energy, 2016, 41(45): 20439 doi: 10.1016/j.ijhydene.2016.08.119 [37] Han B, Mo J K, Kang Z Y, et al. Effects of membrane electrode assembly properties on two-phase transport and performance in proton exchange membrane electrolyzer cells. Electrochimica Acta, 2016, 188: 317 doi: 10.1016/j.electacta.2015.11.139 [38] Bromberger K, Ghinaiya J, Lickert T, et al. Hydraulic ex situ through-plane characterization of porous transport layers in PEM water electrolysis cells. Int J Hydrog Energy, 2018, 43(5): 2556 doi: 10.1016/j.ijhydene.2017.12.042 [39] St?hler M, St?hler A, Scheepers F, et al. Impact of porous transport layer compression on hydrogen permeation in PEM water electrolysis. Int J Hydrog Energy, 2020, 45(7): 4008 doi: 10.1016/j.ijhydene.2019.12.016 [40] Immerz C, Schweins M, Trinke P, et al. Experimental characterization of inhomogeneity in current density and temperature distribution along a single-channel PEM water electrolysis cell. Electrochimica Acta, 2018, 260: 582 doi: 10.1016/j.electacta.2017.12.087 [41] Olesen A C, Frensch S H, K?r S K. Towards uniformly distributed heat, mass and charge: A flow field design study for high pressure and high current density operation of PEM electrolysis cells. Electrochimica Acta, 2019, 293: 476 doi: 10.1016/j.electacta.2018.10.008 [42] Parra-Restrepo J, Bligny R, Dillet J, et al. Influence of the porous transport layer properties on the mass and charge transfer in a segmented PEM electrolyzer. Int J Hydrog Energy, 2020, 45(15): 8094 doi: 10.1016/j.ijhydene.2020.01.100 [43] Matsushima H, Kiuchi D, Fukunaka Y. Measurement of dissolved hydrogen supersaturation during water electrolysis in a magnetic field. Electrochimica Acta, 2009, 54(24): 5858 doi: 10.1016/j.electacta.2009.05.044 [44] Tanaka Y, Kikuchi K, Saihara Y, et al. Bubble visualization and electrolyte dependency of dissolving hydrogen in electrolyzed water using Solid-Polymer-Electrolyte. Electrochimica Acta, 2005, 50(25-26): 5229 doi: 10.1016/j.electacta.2005.01.062 [45] Paliwal S, Panda D, Bhaskaran S, et al. Lattice Boltzmann method to study the water-oxygen distributions in porous transport layer (PTL) of polymer electrolyte membrane (PEM) electrolyser. Int J Hydrog Energy, 2021, 46(44): 22747 doi: 10.1016/j.ijhydene.2021.04.112 [46] Zinser A, Papakonstantinou G, Sundmacher K. Analysis of mass transport processes in the anodic porous transport layer in PEM water electrolysers. Int J Hydrog Energy, 2019, 44(52): 28077 doi: 10.1016/j.ijhydene.2019.09.081 [47] Omrani R, Shabani B. Hydrogen crossover in proton exchange membrane electrolysers: The effect of current density, pressure, temperature, and compression. Electrochimica Acta, 2021, 377: 138085 doi: 10.1016/j.electacta.2021.138085 [48] Trinke P, Bensmann B, Hanke-Rauschenbach R. Experimental evidence of increasing oxygen crossover with increasing current density during PEM water electrolysis. Electrochem Commun, 2017, 82: 98 doi: 10.1016/j.elecom.2017.07.018 -





 下載:
下載: