Effects of graphene oxide doping content and pH on energy storage performance of graphene aerogel
-
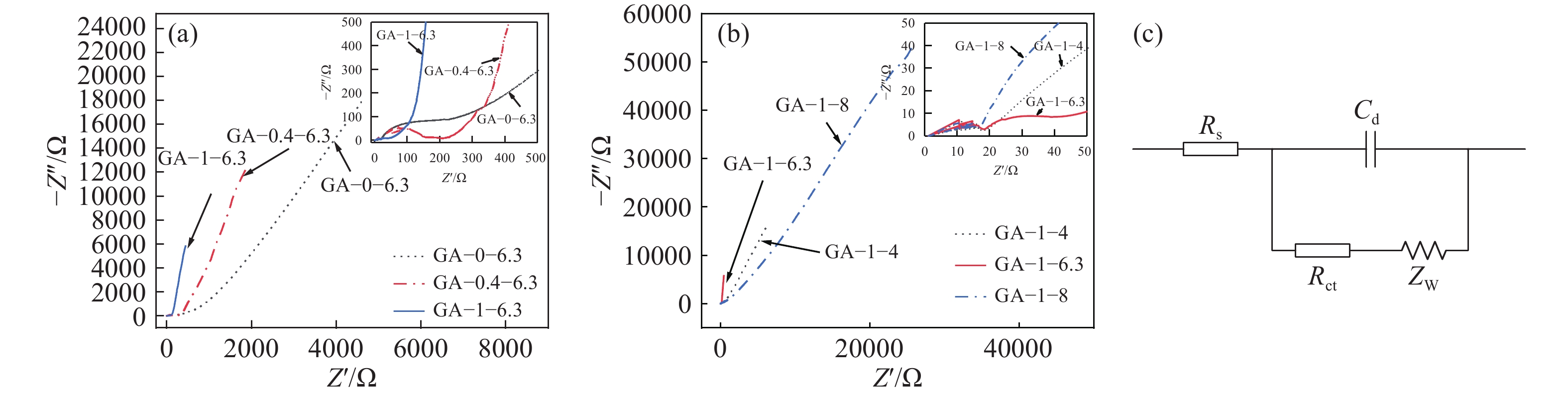
摘要: 采用溶膠凝膠法制備石墨烯氣凝膠(GA),并研究了前驅液中的pH值與氧化石墨烯(GO)的質量分數對GA材料儲能性能的影響。使用X射線粉末衍射(XRD)、X射線光電子能譜(XPS)、氮氣吸脫附分析、掃描電子顯微鏡(SEM)對樣品微觀結構與形貌進行表征。用循環伏安(CV)、恒流充放電(CP)、電化學交流阻抗(EIS)測試了樣品的電化學性能。結果表明,前驅液中的pH值及GO質量分數的不同會影響GA中團簇顆粒的大小和數量,進一步影響GA三維結構。在pH值為6.3、GO 的質量分數為1%時,制得的GA比表面積最大為530 m2?g?1,在1 A?g?1的電流密度下比電容高達364 F?g?1。此外,將該材料制成對稱超級電容器具有高的庫倫效率,在1 A?g?1下進行CP測試,得到電容器的比電容為98 F?g?1,循環800次后其循環穩定性能為初始比電容值的95.9%。Abstract: The preparation of graphene aerogel (GA) by the sol-gel method has wide application prospects. In this study, the sol-gel method was used to prepare GA composites using resorcinol (R), formaldehyde (F), and graphene oxide (GO) as precursor materials and sodium carbonate (C) as the catalyst. The effects of the pH value and GO in the precursor solution on the energy storage performance of GA materials were studied. The microstructure and morphology of the samples were characterized by X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS), nitrogen desorption analysis, and scanning electron microscopy (SEM). Cyclic voltammetry (CV), constant current charge–discharge (CP) and electrochemical impedance spectroscopy (EIS) were used to measure the electrochemical properties of the samples in 1 mol?L?1 Na2SO4 electrolyte. The results show that different pH values and GO affect the size and number of cluster particles in GA and the three-dimensional structure of GA. When the pH value was 6.3 and the mass fraction of GO was 1% in the precursor solution, the obtained GA sample exhibited superior surface properties and electrochemical performance. At a current density of 1 A?g?1, the specific surface area of the GA was 530 m2?g?1, and the specific capacitance was 364 F?g?1. If the current density was increased to 10 A?g?1, the specific capacitance still reached 229 F?g?1, indicating that the GA sample had better multiplier performance. After 800 cycles at a current density of 1 A?g?1, the specific capacitance retention rate was 76%. In addition, the GA sample was utilized as a symmetrical supercapacitor with high coulomb efficiency. The specific capacitance of the capacitor remained at 98 F?g?1 in a constant current charge–discharge test at a current density of 1 A?g?1. After 800 cycles, a specific capacitance retention rate of 95.9% was maintained by the symmetrical supercapacitor. This study provides a method for improving the electrochemical properties of GA to realize supercapacitors with better performance.
-
Key words:
- graphene oxide /
- graphene aerogel /
- pH /
- supercapacitor /
- electrochemical performance
-
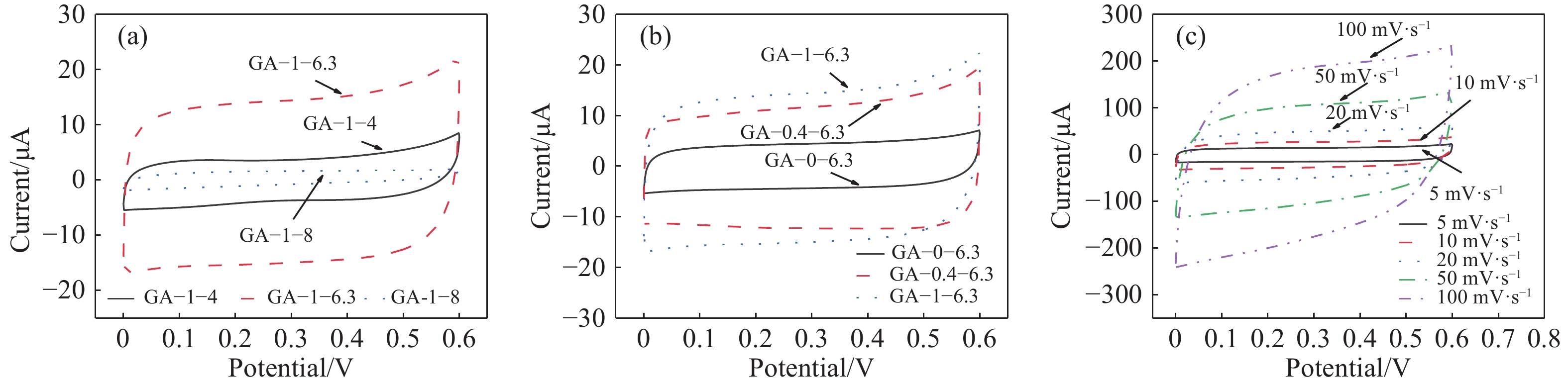
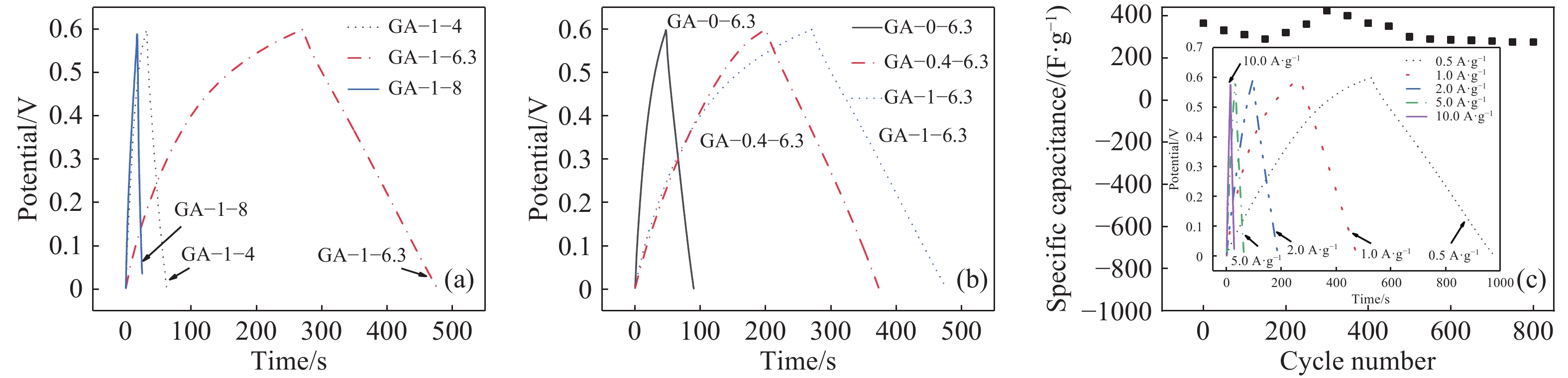
圖 7 GA的CP曲線圖。(a),(b) 1 A?g?1電流密度下五組樣品的CP曲線圖;(c)樣品GA–1–6.3在1 A?g?1電流密度下的循環壽命曲線圖(插圖為不同電流密度下樣品GA–1–6.3的CP曲線圖)
Figure 7. CP curves of GA: (a), (b) CP curves of sample at 1 A?g?1 current density; (c) cycle life curve of GA–1–6.3 at a current density of 1 A?g?1 (inset: CP curves of GA–1–6.3 at different current densities)
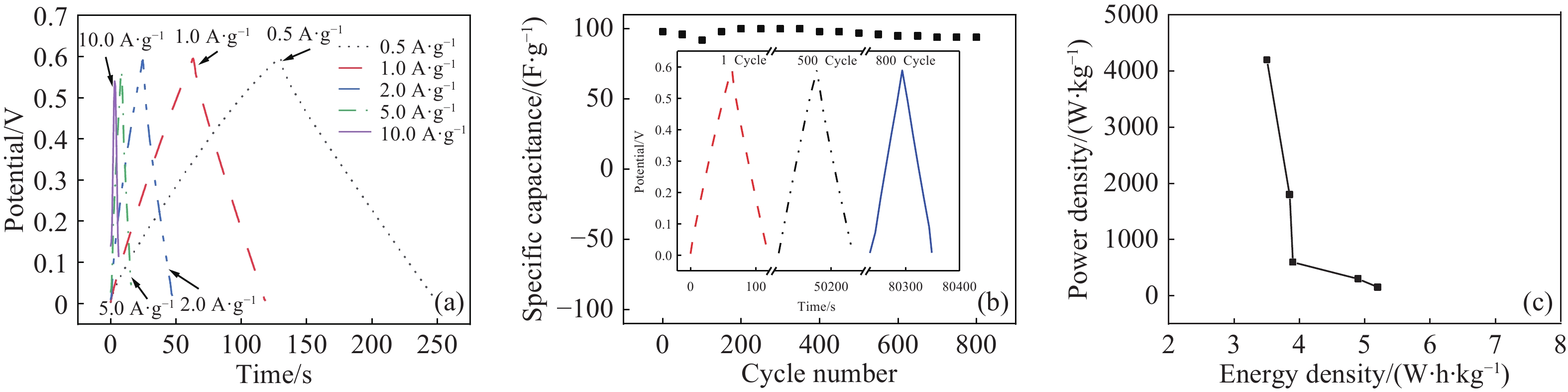
圖 9 超級電容器的CP圖以及其Ragone圖。(a)不同電流密度下超級電容器的CP曲線圖;(b)在1 A?g?1電流密度下超級電容器的循環壽命曲線圖(插圖為超級電容器在1 A?g?1電流密度下第1次、第500次、第800次的CP曲線圖);(c)能量密度與功率密度的曲線
Figure 9. CP curves and Ragone plot of the supercapacitors: (a) CP curves of the supercapacitors at different current densities; (b) cycle life curves of the supercapacitors at a current density of 1 A?g?1 (inset: CP curves of the first, 500th, and 800th cycles of the super capacitors at 1 A?g?1 current density); (c) curve of energy density vs power density
久色视频表 1 五組樣品的比表面積
Table 1. Specific surface areas of the samples
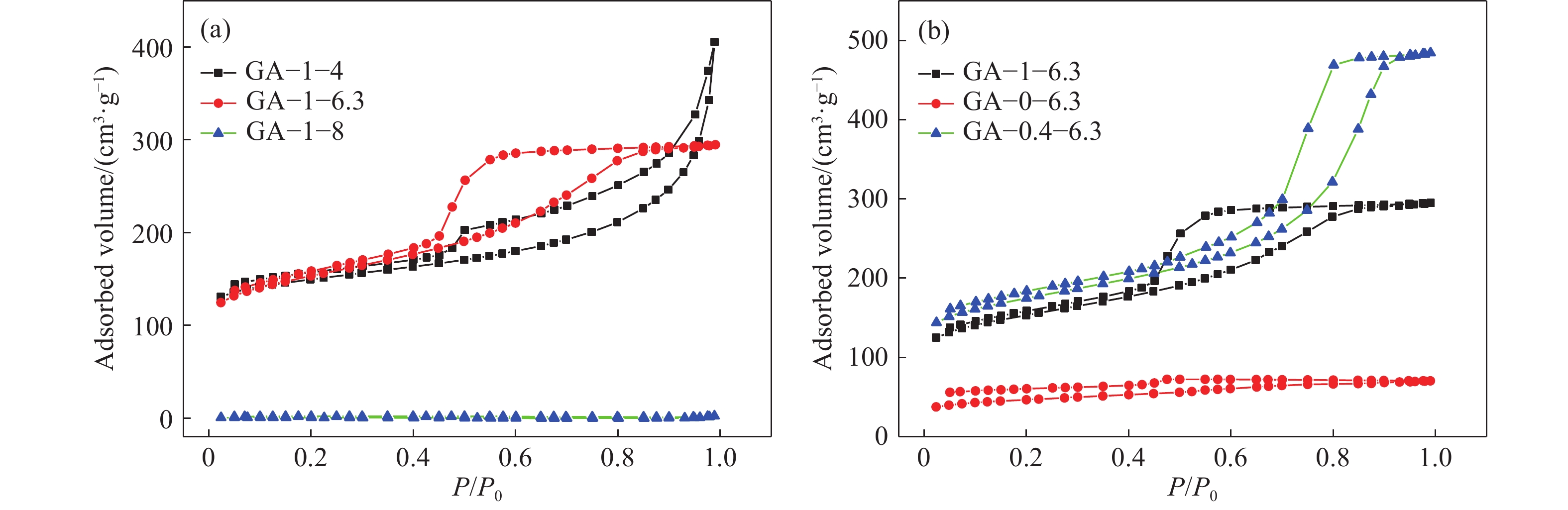
Sample Mass fraction of GO/% pH BET/(m2?g?1) GA–1–4 1 4 231 GA–0–6.3 0 6.3 115 GA–0.4–6.3 0.4 6.3 370 GA–1–6.3 1 6.3 530 GA–1–8 1 8 203 -
參考文獻
[1] Pekala R W. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J Mater Sci, 1989, 24(9): 3221 doi: 10.1007/BF01139044 [2] Liu N, Zhang S T, Fu R W, et al. Carbon aerogel spheres prepared via alcohol supercritical drying. Carbon, 2006, 44(12): 2430 doi: 10.1016/j.carbon.2006.04.032 [3] Xie T P, Zhang L, Wang Y, et al. Graphene-based supercapacitors as flexible wearable sensor for monitoring pulse-beat. Ceram Int, 2019, 45(2): 2516 doi: 10.1016/j.ceramint.2018.10.181 [4] Chandrasekaran S, Campbell P G, Baumann T F, et al. Carbon aerogel evolution: allotrope, graphene-inspired, and 3D-printed aerogels. J Mater Res, 2017, 32(22): 4166 doi: 10.1557/jmr.2017.411 [5] Novoselov K S, Geim A K, Morozov S V, et al. Electric field effect in atomically thin carbon films. Science, 2004, 306(5696): 666 doi: 10.1126/science.1102896 [6] Liu P P, Liu S Q, Gao H Y, et al. Preparation and properties of hydroxyapatite aerogel composite phase change materials. Chin J Eng, 2020, 42(1): 120劉盼盼, 劉斯奇, 高鴻毅, 等. 羥基磷灰石氣凝膠復合相變材料的制備及其性能. 工程科學學報, 2020, 42(1):120 [7] Wang Y X, Myers M, Staser J A. Electrochemical UV sensor using carbon quantum dot/graphene semiconductor. J Electrochem Soc, 2018, 165(4): H3001 doi: 10.1149/2.0011804jes [8] Shui L, Zhang K, Yu H. Effect of graphene content on the microstructure and mechanical properties of graphene-reinforced Al–15Si–4Cu–Mg matrix composites. Chin J Eng, 2019, 41(9): 1162水麗, 張凱, 于宏. 石墨烯含量對石墨烯/Al–15Si–4Cu–Mg復合材料微觀組織和力學性能的影響. 工程科學學報, 2019, 41(9):1162 [9] Méndez-Morales T, Ganfoud N, Li Z J, et al. Performance of microporous carbon electrodes for supercapacitors: comparing graphene with disordered materials. Energy Storage Mater, 2019, 17: 88 doi: 10.1016/j.ensm.2018.11.022 [10] Fu R R, Luo M, Ma Y H, et al. Preparation and supercapacitance of Ni3(HCOO)6/reduced graphene oxide electrode materials. Chem J Chin Univ, 2016, 37(8): 1485 doi: 10.7503/cjcu20160234付蓉蓉, 羅民, 馬永華, 等. Ni3(HCOO)6/還原氧化石墨烯復合電極材料的制備及電容性能. 高等學校化學學報, 2016, 37(8):1485 doi: 10.7503/cjcu20160234 [11] Zou Z H, Zhou W J, Zhang Y H, et al. High-performance flexible all-solid-state supercapacitor constructed by free-standing cellulose/reduced graphene oxide/silver nanoparticles composite film. Chem Eng J, 2019, 357: 45 doi: 10.1016/j.cej.2018.09.143 [12] Wu X F, Zhang J, Zhuang Y F, et al. Template-free preparation of a few-layer graphene nanomesh via a one-step hydrothermal process. J Mater Sci, 2015, 50(3): 1317 doi: 10.1007/s10853-014-8691-4 [13] Zhang J J, Zhao X L, Li M X, et al. High-quality and low-cost three-dimensional graphene from graphite flakes via carbocation-induced interlayer oxygen release. Nanoscale, 2018, 10(37): 17638 doi: 10.1039/C8NR04557G [14] Gao X. Fabrication and Electrochemical Properties of the Graphene Based Composites as Supercapacitor Electrode Materials [Dissertation]. Harbin: Harbin University of Science and Technology, 2019高鑫. 石墨烯基超級電容器電極材料的制備及電化學性能[學位論文]. 哈爾濱: 哈爾濱理工大學, 2019 [15] Xu X, Zhang Q Q, Yu Y K, et al. Naturally dried graphene aerogels with superelasticity and tunable Poisson’s ratio. Adv Mater, 2016, 28(41): 9223 doi: 10.1002/adma.201603079 [16] González M, Baselga J, Pozuelo J. Modulating the electromagnetic shielding mechanisms by thermal treatment of high porosity graphene aerogels. Carbon, 2019, 147: 27 doi: 10.1016/j.carbon.2019.02.068 [17] Xue Q, Ding Y, Xue Y Y, et al. 3D nitrogen-doped graphene aerogels as efficient electrocatalyst for the oxygen reduction reaction. Carbon, 2018, 139: 137 doi: 10.1016/j.carbon.2018.06.052 [18] Chu H, Zhang F F, Pei L Y, et al. Ni, Co and Mn doped SnS2-graphene aerogels for supercapacitors. J Alloys Compd, 2018, 767: 583 doi: 10.1016/j.jallcom.2018.07.126 [19] Ates M, Caliskan S, Ozten E. Preparation of rGO/Ag/PEDOT nanocomposites for supercapacitors. Mater Technol, 2018, 33(14): 872 doi: 10.1080/10667857.2018.1521087 [20] Yang Y, Xi Y L, Li J Z, et al. Flexible supercapacitors based on polyaniline arrays coated graphene aerogel electrodes. Nanoscale Res Lett, 2017, 12: 394 doi: 10.1186/s11671-017-2159-9 [21] Liu L, Tian G Y, Ma R, et al. Preparation and electrosorption performance of graphene Xerogel. ECS Solid State Lett, 2015, 4(6): M9 doi: 10.1149/2.0011506ssl [22] Nagy B, Bakos I, Bertoti I, et al. Synergism of nitrogen and reduced graphene in the electrocatalytic behavior of resorcinol - Formaldehyde based carbon aerogels. Carbon, 2018, 139: 872 doi: 10.1016/j.carbon.2018.07.061 [23] Rey-Raap N, Arenillas A, Menendez J A. A visual validation of the combined effect of pH and dilution on the porosity of carbon xerogels. Microporous Mesoporous Mater, 2016, 223: 89 doi: 10.1016/j.micromeso.2015.10.044 [24] Garcia-Bordeje E, Victor-Roman S, Sanahuja-Parejo O, et al. Control of the microstructure and surface chemistry of graphene aerogels via pH and time manipulation by a hydrothermal method. Nanoscale, 2018, 10(7): 3526 doi: 10.1039/C7NR08732B [25] Horikaw T, Hayashi J, Muroyama K. Controllability of pore characteristics of resorcinol–formaldehyde carbon aerogel. Carbon, 2004, 42(8-9): 1625 doi: 10.1016/j.carbon.2004.02.016 [26] Feng Y N, Wang J, Ge L, et al. Pore size controllable preparation for low density porous nano-carbon. J Nanosci Nanotechnol, 2013, 13(10): 7012 doi: 10.1166/jnn.2013.8063 [27] Rey-Raap N, Menendez J A, Arenillas A. Simultaneous adjustment of the main chemical variables to fine-tune the porosity of carbon xerogels. Carbon, 2014, 78: 490 doi: 10.1016/j.carbon.2014.07.030 [28] Elkhatat A M, Al-Muhtaseb S A. Advances in tailoring resorcinol-formaldehyde organic and carbon gels. Adv Mater, 2011, 23(26): 2887 doi: 10.1002/adma.201100283 [29] Gallegos-Suarez E, Perez-Cadenas A F, Maldonado-Hodar F J, et al. On the micro- and mesoporosity of carbon aerogels and xerogels. The role of the drying conditions during the synthesis processes. Chem Eng J, 2012, 181-182: 851 doi: 10.1016/j.cej.2011.12.002 [30] Al-Muhtaseb S A, Ritter J A. Preparation and properties of resorcinol–formaldehyde organic and carbon gels. Adv Mater, 2003, 15(2): 101 doi: 10.1002/adma.200390020 [31] Matos I, Fernandes S, Guerreiro L, et al. The effect of surfactants on the porosity of carbon xerogels. Microporous Mesoporous Mater, 2006, 92(1-3): 38 doi: 10.1016/j.micromeso.2005.12.011 [32] Rey-Raap N, Menendez J A, Arenillas A. RF xerogels with tailored porosity over the entire nanoscale. Microporous Mesoporous Mater, 2014, 195: 266 doi: 10.1016/j.micromeso.2014.04.048 [33] Xia X H, Zhang X F, Yi S Q, et al. Preparation of high specific surface area composite carbon cryogels from self-assembly of graphene oxide and resorcinol monomers for supercapacitors. J Solid State Electrochem, 2016, 20(6): 1793 doi: 10.1007/s10008-016-3196-5 [34] Wu Z S, Ren W C, Wang D W, et al. High-energy MnO2 nanowire/graphene and graphene asymmetric electrochemical capacitors. ACS Nano, 2010, 4(10): 5835 doi: 10.1021/nn101754k -




 下載:
下載: