Research progress of MOFs-derived materials as the electrode for lithium–ion batteries — a short review
-
摘要: 金屬有機框架材料 (Metal-organic frameworks,MOFs)是一種新穎的多孔晶體材料,具有比表面積大、孔隙率高、結構可設計性強等優點,但是,MOFs的低電導率以及在電解液中的穩定性等問題限制了其作為電極材料的應用。近年來,如何結合MOFs的優勢進行鋰離子電池電極材料的設計與合成受到了越來越多的關注。目前,通過自犧牲得到的多孔碳骨架和金屬化合物等MOFs衍生復合電極材料,不僅解決了電導率低的問題,而且保留了MOFs的高比表面積和復雜多孔結構,為鋰離子的插入/脫出、吸附/解吸等過程提供了豐富的活性位點;與此同時,從結構單元和化學組成方面增加了材料結構的復雜性,開放性的孔隙結構可以緩沖體積膨脹帶來的機械應力,對外來離子存儲和多離子傳輸具有重要的意義。本文綜述了MOFs及其衍生物在鋰離子電池電極材料的設計和研究中取得的最新進展,重點闡述了針對鋰離子電池電極材料的要求進行MOFs形貌控制和修飾的方法,以及具有多孔、中空或特殊結構的MOFs衍生電極材料的制備關鍵影響因素及其結構特性對電化學性能的影響。最后,分析了MOFs衍生電極材料的研究挑戰和發展方向。Abstract: Owing to their high surface area, excellent electrolyte permeability and ample diffusion pathways for charge transport, porous and hollow-structured electrochemically active materials attract more attention as the electrodes. In general, the process of template preparation method is used to achieve hollow structured materials over the last few decades. However, the complicated preparation process including removal of template and surface modification often results in poor uniformity, low reproducibility, and high cost of porous structure. Moreover, it incorporates functional chemicals with specific homogeneity and dispersity into the hollow porous intercrystalline structure. These problems hinder the development and application in energy storage and conversion devices of the diversified porous and hollow-structured materials. The metal-organic frameworks (MOFs), consisting of organic linkers and coordinated inorganic clusters, appear as an excellent collection of porous crystal material series with high surface areas, high porosity, and tunable structures. However, their low conductivity and electrolyte instability limit the further use of MOFs in the field of LIBs. Recently, how electrode materials for Lithium–ion batteries (LIBs) are designed and prepare using MOFs has attracted more attention. The composite materials derived from MOFs including nanostructured porous carbons and metal oxide uaing self-sacrificial template synthetic route not only solves the problem of low conductivity but also maintains the high surface area and porous structure of MOFs, providing abundant active sites for insertion/deinsertion or adsorption/desorption; Furthermore, composite materials derived from MOFs increase the complexity of nanostructures in terms of structural units and chemical components. In particular, large pore volume and open pore structure are critical to loading guest species, accommodating mechanical strains and facilitating mass transport. In this paper, we briefly examined the production of MOF-derived materials for applications in LIBs. The optimization and modification of an MOFs morphology were implemented according to the electrode material requirement for LIBs. Moreover, the preparation of MOFs-derived electrode materials with porous, hollow, or complicated construction and their effects on electrochemical performance were described. Finally, the challenge and trend in production of electrode materials derived from MOFs were analyzed.
-
Key words:
- lithium ion battery /
- electrode material /
- metal organic frameworks /
- porous structure /
- cycle life
-
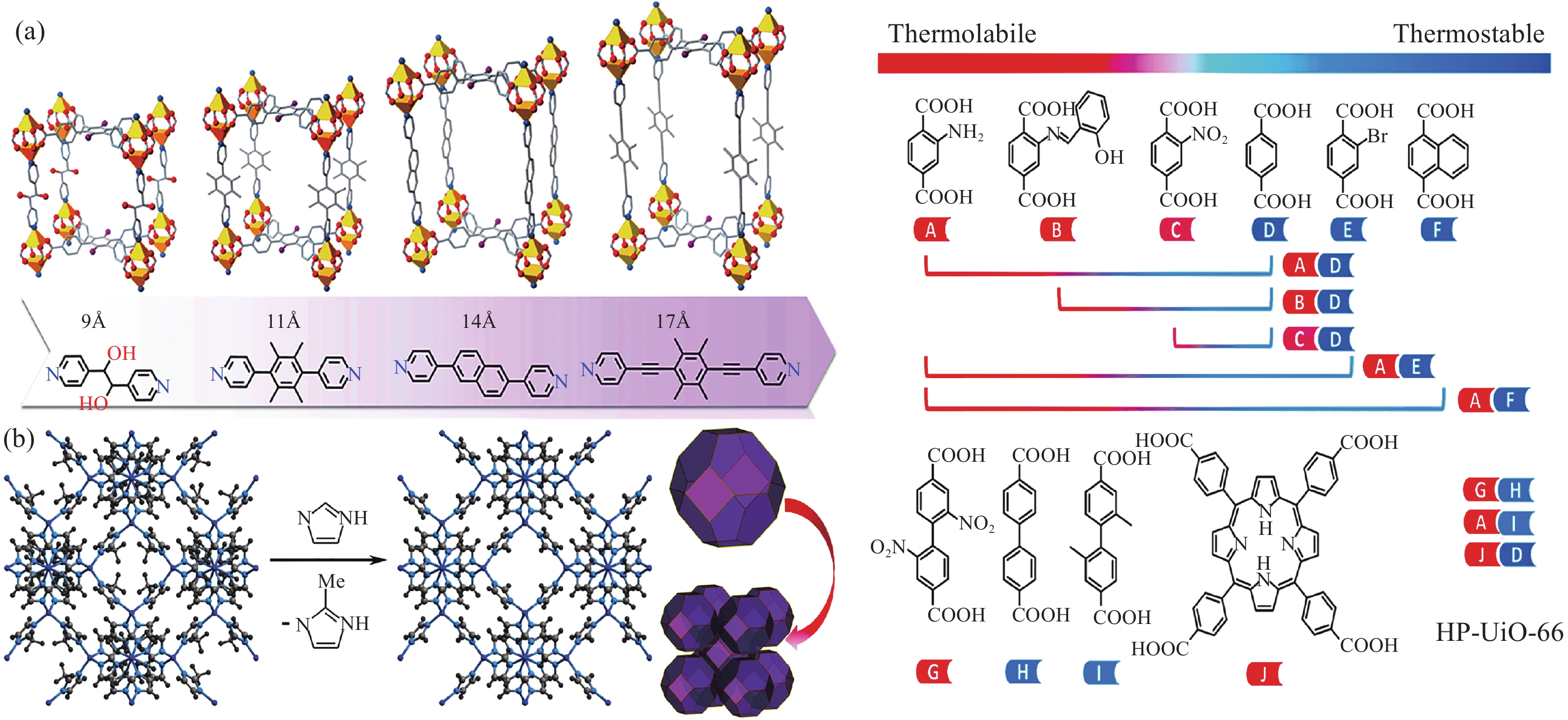
圖 2 不同尺寸的聯吡啶基有機配體合成金屬有機框架的示意圖(a)[18];ZIF?8經過溶劑輔助更換配體后孔徑擴張示意圖(b)[20];利用不同熱穩定性的連接體制備HP?UiO?66的示意圖(c)(A~J代表著不同配體的熱穩定性)[21]
Figure 2. Schematic diagram (a) of the synthesis of metal organic frames using different sizes of bipyridyl organic ligands[18]; aperture expansion in ZIF?8 via solvent-assisted linker exchange (b)[20]; versatility of linker thermolysis to construct HP?UiO?66 using various linkers (c) (A–J showing different thermal stability)[21]
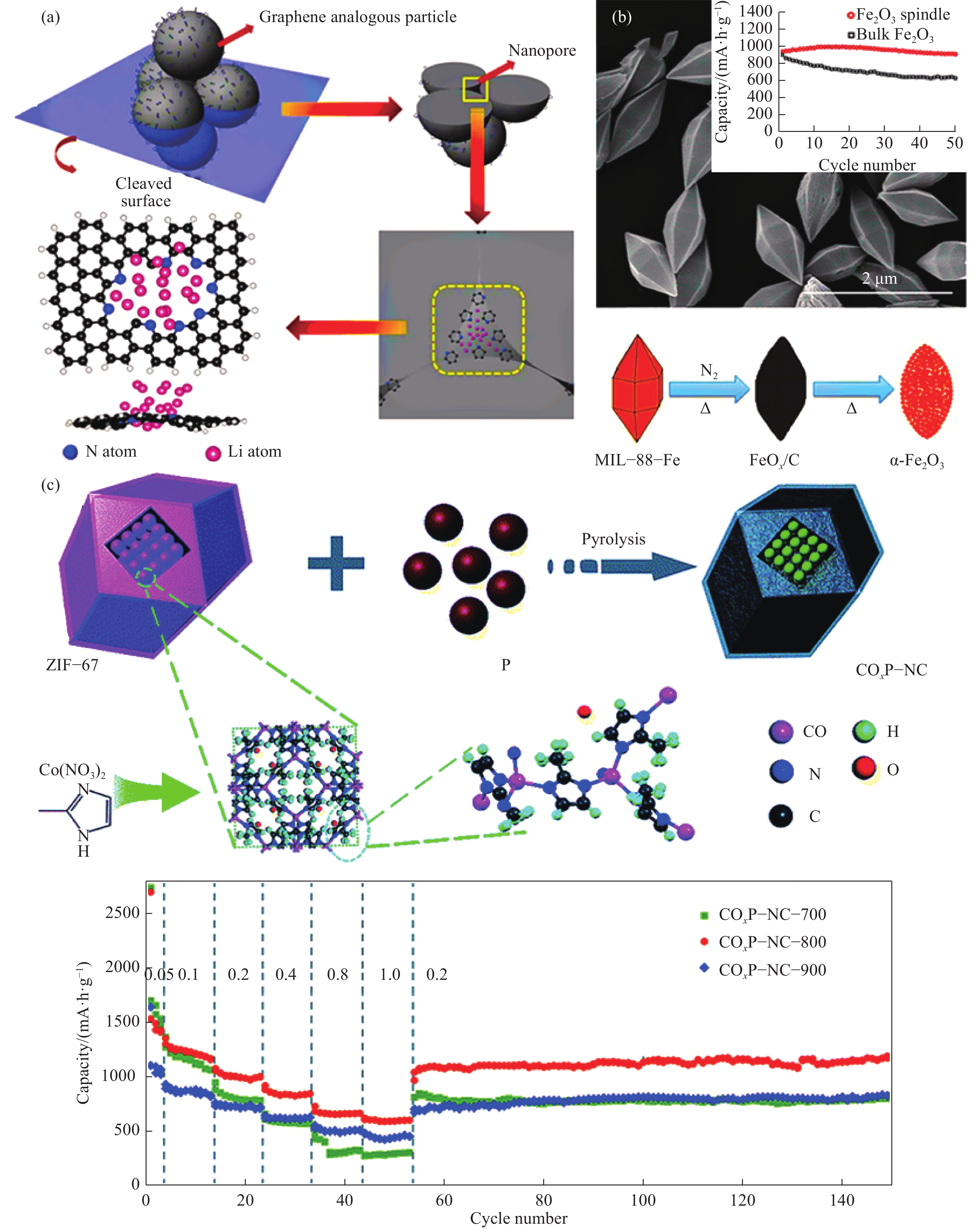
圖 4 具有豐富氮摻雜的ZIF?8衍生碳粒中額外鋰離子存儲機理示意圖(a)[36];MIL?88?Fe的掃描電鏡圖,合成紡錘狀多孔α-Fe2O3的流程圖,以及在200 mA·g?1電流密度下紡錘狀多孔α-Fe2O3和塊狀Fe2O3循環性能對比(嵌入圖)(b)[46];合成CoxP?NC多面體的流程示意圖,以及CoxP?NC?700, CoxP?NC?800和CoxP?NC?900電極材料倍率性能的對比圖(c)[47]
Figure 4. Schematic representation (a) of extra Li storage in N-doped ZIF?8 derived carbon particles[36]; SEM image of as-prepared MIL?88?Fe, the illustration of the fabrication of spindle-like porous α-Fe2O3, and comparative cycling performance of the final spindle-like α-Fe2O3 and bulk Fe2O3 at 200 mA·g?1 (inset) (b)[46]; Schematic illustration of the formation of CoxP?NC polyhedra, and rate performance of the CoxP?NC?700, CoxP?NC?800 and CoxP?NC?900 electrodes at different rate current densities (c)[47]
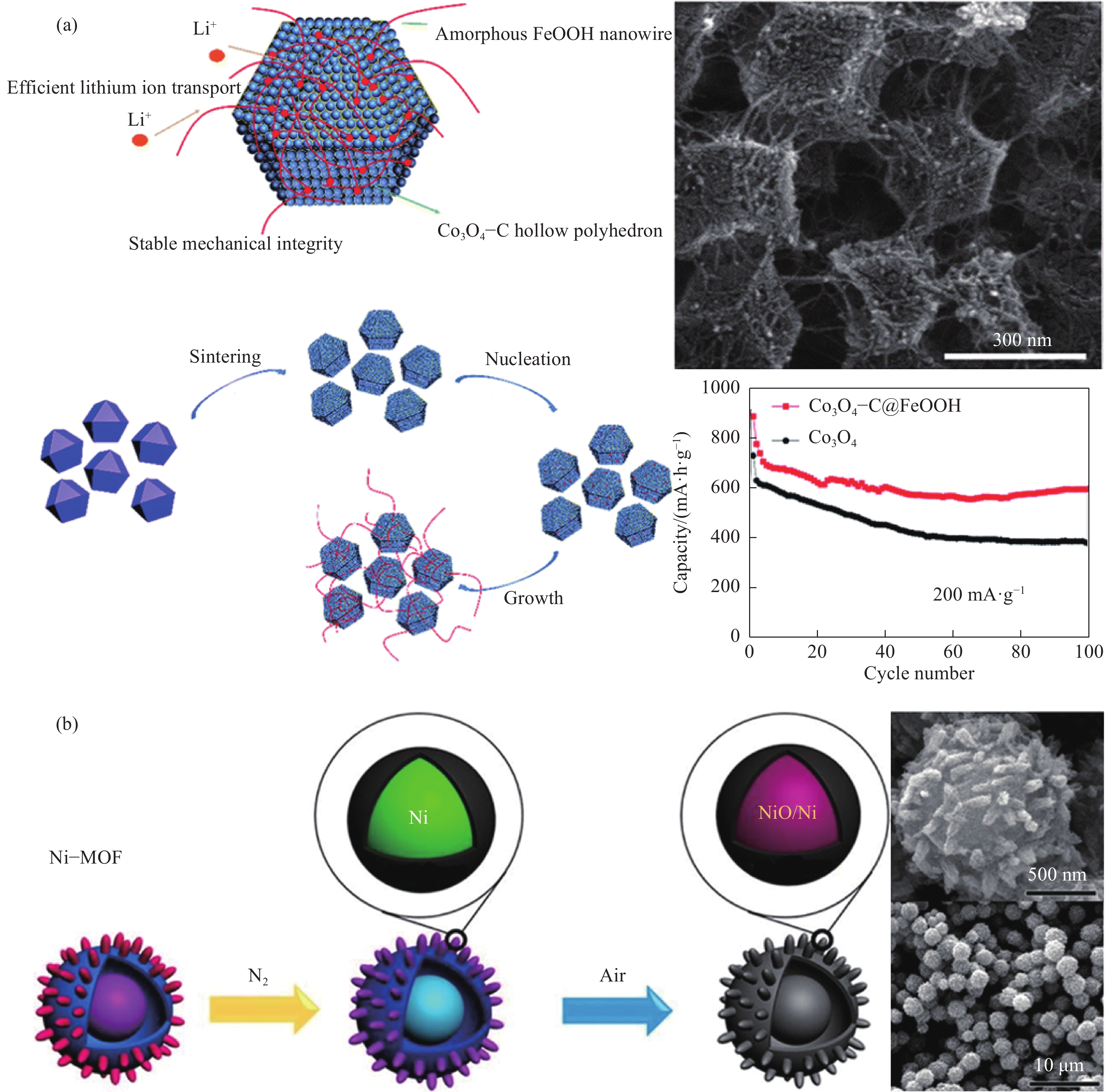
圖 5 交織異質結構示意圖,Co3O4?C@FeOOH交織中空多面體結構的形成過程,以及微觀形貌和循環性能對比圖(a)[56];NiO/Ni/石墨烯復合材料合成示意圖,以及NiO/Ni/石墨烯復合材料的掃描電鏡圖(b)[59]。
Figure 5. Schematic of interwoven heterostructure, schematic showing the formation process of crystalline–amorphous Co3O4/FeOOH interwoven hollow polyhedrons structure, and SEM image and cycling performance (a)[56]; Schematic of the formation of NiO/Ni/Graphene composites, and SEM images of NiO/Ni/Graphene composite (b)[59]
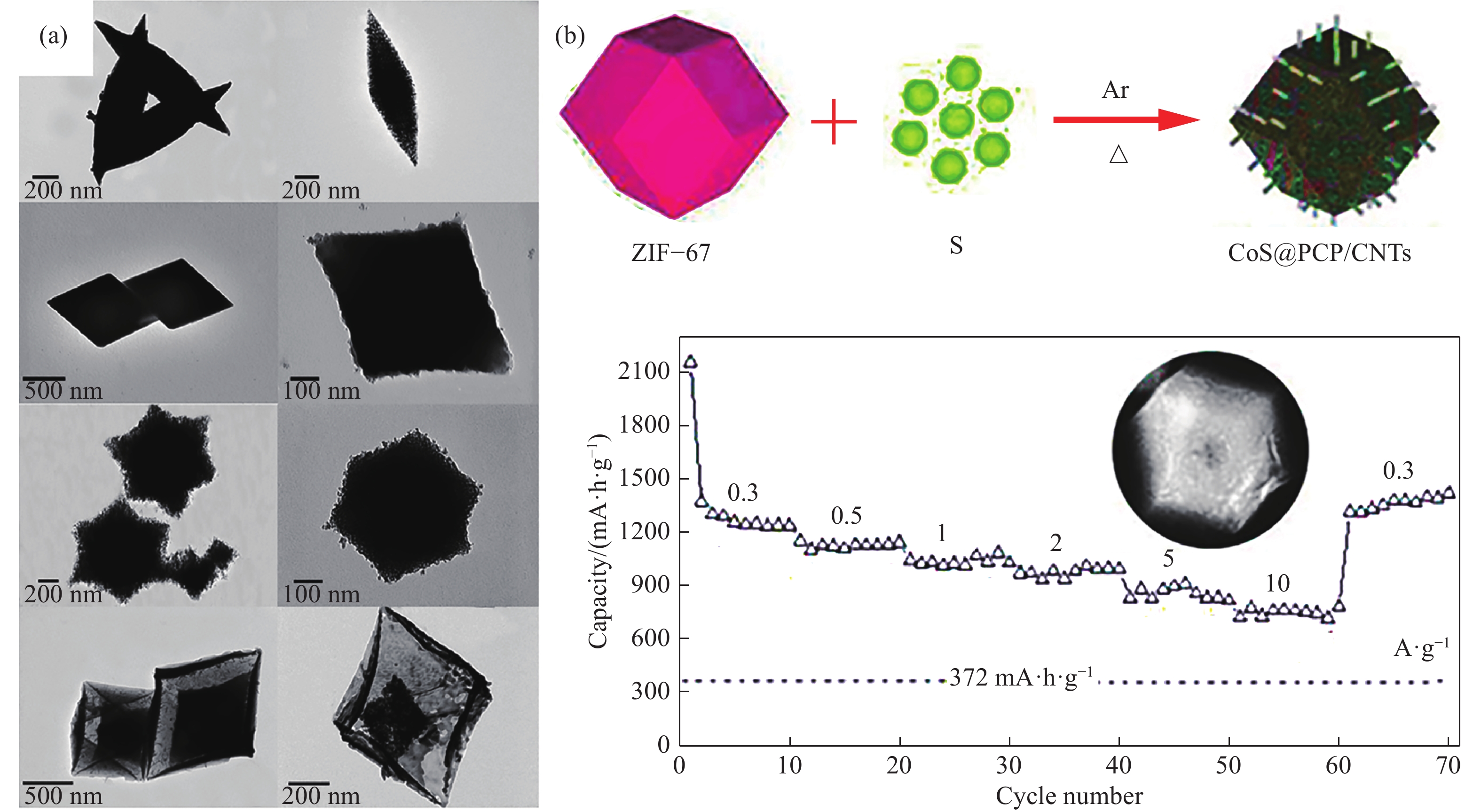
圖 6 鐵基MOFs及其衍生的Fe2O3納米結構的掃描電鏡圖像(a)[68];3D中空CoS@PCP/CNTs的合成示意圖,以及0.3?10 A·g?1不同電流密度下CoS@PCP/CNTs的放電比容量(b)[69]。
Figure 6. SEM images (a) of Fe-based MOFs and their derived Fe2O3 nanostructures[68]; schematic for the formation of 3D hollow CoS@PCP/CNTs, and rate capabilities of CoS@PCP/CNTs at various current densities between 0.3 and 10 A·g?1 (b)[69]
表 1 熱解后得到的MOFs材料HP?UiO?66的孔隙參數[21]
Table 1. Porosity parameters of HP?UiO?66 created by linker thermolysis[21]
Sample SBET/(cm3·g?1) Dmeso/nm V(meso)/V(micro) HP?UiO?66?AD 1022 9.8 0.83 HP?UiO?66?BD 1012 7.5 0.60 HP?UiO?66?CD 825 7.2 0.82 HP?UiO?66?AE 702 5.5 0.79 HP?UiO?66?AF 571 6.0 1.00 HP?UiO?67?GH 2185 14.8 0.66 表 2 幾種以MOFs為前驅體制備的多孔納米碳基電極材料
Table 2. Some porous carbon-based electrode nanomaterials prepared using MOFs
表 3 幾種以MOFs為前驅體制備的多孔金屬化合物或金屬化合物/碳復合電極材料
Table 3. Some porous metal compounds or metal compound/carbon composite electrode materials prepared using MOFs
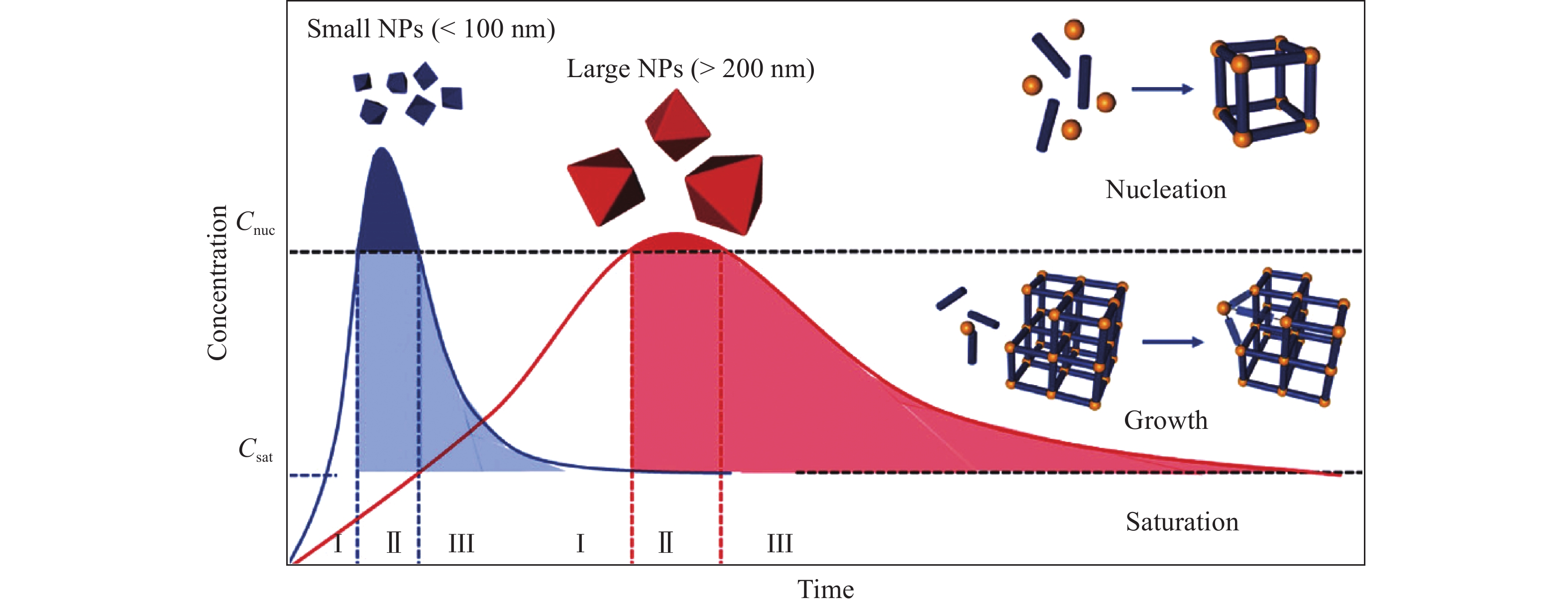
久色视频表 4 幾種以MOFs為前驅體制備的具有中空結構的復合電極材料
Table 4. Some hollow composite electrode materials prepared using MOFs
Sample Precursor Capacity/(mA·h·g?1) Cycle number Voltage / (V vs Li/Li+) Current density /(mA·g?1) Reference Multishell microsphere Co3O4@C Ni/Co?MOF 1701 60 0.01?3 100 [60] CoSe@C nanoboxes ZIF?67 787 100 0.01?3 200 [61] Hollow CoS2 ZIF?8/67 549.9 200 0.01?3 1000 [62] Hollow Fe2O3/SnO2 PB 500 100 0.05?3 200 [63] Double-Shelled Nanocages CH@LDH ZIF?67 653 100 0.01?3 65 [64] Microboxes Fe2O3 PB 950 30 0.01?3 200 [65] Nanobubble Hollow CoS2 ZIF?67 737 200 0.05?3 1000 [66] Nanobowls CMS/NSC NB 218.6 8240 0.01?2.5 5000 [67] -
參考文獻
[1] Auvergniot J, Cassel A, Ledeuil J B, et al. Interface stability of argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in bulk all-solid-state batteries. Chem Mater, 2017, 29(9): 3883 doi: 10.1021/acs.chemmater.6b04990 [2] Gong Y, Zhang J N, Jiang L W, et al. In situ atomic-scale observation of electrochemical delithiation induced structure evolution of LiCoO2 cathode in a working all-solid-state battery. J Am Chem Soc, 2017, 139(12): 4274 doi: 10.1021/jacs.6b13344 [3] Konarov A, Myung S T, Sun Y K. Cathode materials for future electric vehicles and energy storage systems. ACS Energy Lett, 2017, 2(3): 703 doi: 10.1021/acsenergylett.7b00130 [4] Tron A, Jo Y N, Oh S H, et al. Surface modification of the LiFePO4 cathode for the aqueous rechargeable lithium ion battery. ACS Appl Mater Interfaces, 2017, 9(14): 12391 doi: 10.1021/acsami.6b16675 [5] Capasso C, Veneri O. Experimental analysis on the performance of lithium based batteries for road full electric and hybrid vehicles. Appl Energy, 2014, 136: 921 doi: 10.1016/j.apenergy.2014.04.013 [6] Gao X P, Yang H X. Multi-electron reaction materials for high energy density batteries. Energy Environ Sci, 2010, 3(2): 174 doi: 10.1039/B916098A [7] Bruce P G, Freunberger S A, Hardwick L J, et al. Li?O2 and Li?S batteries with high energy storage. Nat Mater, 2012, 11: 19 doi: 10.1038/nmat3191 [8] Zhu Z Q, Wang S W, Du J, et al. Ultrasmall Sn nanoparticles embedded in nitrogen-doped porous carbon as high-performance anode for lithium-ion batteries. Nano Lett, 2014, 14(1): 153 doi: 10.1021/nl403631h [9] Sacci R L, Lehmann M L, Diallo S O, et al. Lithium transport in an amorphous LixSi anode investigated by quasi-elastic neutron scattering. J Phys Chem C, 2017, 121(21): 11083 doi: 10.1021/acs.jpcc.7b01133 [10] Lü X X, Deng J J, Wang B Q, et al. γ-Fe2O3@ CNTs anode materials for lithium ion batteries investigated by electron energy loss spectroscopy. Chem Mater, 2017, 29(8): 3499 doi: 10.1021/acs.chemmater.6b05356 [11] Jiang T C, Bu F X, Feng X X, et al. Porous Fe2O3 nanoframeworks encapsulated within three-dimensional graphene as high-performance flexible anode for lithium-ion battery. ACS Nano, 2017, 11(5): 5140 doi: 10.1021/acsnano.7b02198 [12] Zhou J W, Wang B. Emerging crystalline porous materials as a multifunctional platform for electrochemical energy storage. Chem Soc Rev, 2017, 46(22): 6927 doi: 10.1039/C7CS00283A [13] Howarth A J, Liu Y Y, Li P, et al. Chemical, thermal and mechanical stabilities of metal-organic frameworks. Nat Rev Mater, 2016, 1: 15018 doi: 10.1038/natrevmats.2015.18 [14] Hu L, Chen Q. Hollow/porous nanostructures derived from nanoscale metal-organic frameworks towards high performance anodes for lithium-ion batteries. Nanoscale, 2014, 6(3): 1236 doi: 10.1039/C3NR05192G [15] Chen L Y, Luque R, Li Y W. Controllable design of tunable nanostructures inside metal-organic frameworks. Chem Soc Rev, 2017, 46(15): 4614 doi: 10.1039/C6CS00537C [16] Kim D, Coskun A. Template-directed approach towards the realization of ordered heterogeneity in bimetallic metal-organic frameworks. Angew Chem Int Ed Engl, 2017, 56(18): 5071 doi: 10.1002/anie.201702501 [17] An T C, Wang Y H, Tang J, et al. A flexible ligand-based wavy layered metal-organic framework for lithium-ion storage. J Colloid Interface Sci, 2015, 445: 320 doi: 10.1016/j.jcis.2015.01.012 [18] Weston M H, Delaquil A A, Sarjeant A A, et al. Tuning the hydrophobicity of zinc dipyridyl paddlewheel metal-organic frameworks for selective sorption. Cryst Growth Des, 2013, 13(7): 2938 doi: 10.1021/cg400342m [19] Kirchon A, Feng L, Drake H F, et al. From fundamentals to applications: a toolbox for robust and multifunctional MOF materials. Chem Soc Rev, 2018, 47(23): 8611 doi: 10.1039/C8CS00688A [20] Karagiaridi O, Lalonde M B, Bury W, et al. Opening ZIF-8: a catalytically active zeolitic imidazolate framework of sodalite topology with unsubstituted linkers. J Am Chem Soc, 2012, 134(45): 18790 doi: 10.1021/ja308786r [21] Feng L, Yuan S, Zhang L L, et al. Creating hierarchical pores by controlled linker thermolysis in multivariate metal-organic frameworks. J Am Chem Soc, 2018, 140(6): 2363 doi: 10.1021/jacs.7b12916 [22] Yec C C, Zeng H C. Synthesis of complex nanomaterials via Ostwald ripening. J Mater Chem A, 2014, 2(14): 4843 doi: 10.1039/C3TA14203E [23] Cui Y J, Li B, He H J, et al. Metal-organic frameworks as platforms for functional materials. Acc Chem Res, 2016, 49(3): 483 doi: 10.1021/acs.accounts.5b00530 [24] Wang S Z, McGuirk C M, d'Aquino A, et al. Metal-organic framework nanoparticles. Adv Mater, 2018, 30(37): 1800202 doi: 10.1002/adma.201800202 [25] LaMer V K, Dinegar R H. Theory, production and mechanism of formation of monodispersed hydrosols. J Am Chem Soc, 1950, 72(11): 4847 doi: 10.1021/ja01167a001 [26] Banerjee A, Upadhyay K K, Puthusseri D, et al. MOF-derived crumpled-sheet-assembled perforated carbon cuboids as highly effective cathode active materials for ultra-high energy density Li-ion hybrid electrochemical capacitors (Li-HECs). Nanoscale, 2014, 6(8): 4387 doi: 10.1039/c4nr00025k [27] Fang G Z, Zhou J, Liang C W, et al. MOFs nanosheets derived porous metal oxide-coated three-dimensional substrates for lithium-ion battery applications. Nano Energy, 2016, 26: 57 doi: 10.1016/j.nanoen.2016.05.009 [28] Salunkhe R R, Kaneti Y V, Yamauchi Y. Metal-organic framework-derived nanoporous metal oxides toward supercapacitor applications: progress and prospects. ACS Nano, 2017, 11(6): 5293 doi: 10.1021/acsnano.7b02796 [29] Liu Y S, Shen H B, Jiang H, et al. ZIF-derived graphene coated/Co9S8 nanoparticles embedded in nitrogen doped porous carbon polyhedrons as advanced catalysts for oxygen reduction reaction. Int J Hydrogen Energy, 2017, 42(18): 12978 doi: 10.1016/j.ijhydene.2017.04.050 [30] Zhang Y F, Pan A Q, Wang Y P, et al. Dodecahedron-shaped porous vanadium oxide and carbon composite for high-rate lithium ion batteries. ACS Appl Mater Interfaces, 2016, 8(27): 17303 doi: 10.1021/acsami.6b04866 [31] Xie Z Q, Xu W W, Cui X D, et al. Recent progress in metal-organic frameworks and their derived nanostructures for energy and environmental applications. ChemSusChem, 2017, 10(8): 1645 doi: 10.1002/cssc.201601855 [32] Zhang W, Jiang X F, Zhao Y Y, et al. Hollow carbon nanobubbles: monocrystalline MOF nanobubbles and their pyrolysis. Chem Sci, 2017, 8(5): 3538 doi: 10.1039/C6SC04903F [33] Hu Z W, Zhang Z P, Li Z L, et al. One-step conversion from core-shell metal-organic framework materials to cobalt and nitrogen codoped carbon nanopolyhedra with hierarchically porous structure for highly efficient oxygen reduction. ACS Appl Mater Interfaces, 2017, 9(19): 16109 doi: 10.1021/acsami.7b00736 [34] Jiang Y, Liu H Q, Tan X H, et al. Monoclinic ZIF-8 nanosheet-derived 2D carbon nanosheets as sulfur immobilizer for high-performance lithium sulfur batteries. ACS Appl Mater Interfaces, 2017, 9(30): 25239 doi: 10.1021/acsami.7b04432 [35] Leyssale J M, Vignoles G L. Molecular dynamics evidences of the full graphitization of a nanodiamond annealed at 1500 K. Chem Phys Lett, 2008, 454(4-6): 299 doi: 10.1016/j.cplett.2008.02.025 [36] Zheng F C, Yang Y, Chen Q W. High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal-organic framework. Nat Commun, 2014, 5: 5261 doi: 10.1038/ncomms6261 [37] Li A, Tong Y, Cao B, et al. MOF-derived multifractal porous carbon with ultrahigh lithium-ion storage performance. Sci Rep, 2017, 7: 40574 doi: 10.1038/srep40574 [38] Guo Y Y, Zeng X Q, Zhang Y, et al. Sn nanoparticles encapsulated in 3D nanoporous carbon derived from a metal-organic framework for anode material in lithium-ion batteries. ACS Appl Mater Interfaces, 2017, 9(20): 17172 doi: 10.1021/acsami.7b04561 [39] Zhang P, Sun F, Xiang Z H, et al. ZIF-derived in situ nitrogen-doped porous carbons as efficient metal-free electrocatalysts for oxygen reduction reaction. Energy Environ Sci, 2014, 7(1): 442 doi: 10.1039/C3EE42799D [40] Jiang H L, Liu B, Lan Y Q, et al. From metal-organic framework to nanoporous carbon: toward a very high surface area and hydrogen uptake. J Am Chem Soc, 2011, 133(31): 11854 doi: 10.1021/ja203184k [41] Su P P, Xiao H, Zhao J, et al. Nitrogen-doped carbon nanotubes derived from Zn-Fe-ZIF nanospheres and their application as efficient oxygen reduction electrocatalysts with in situ generated iron species. Chem Sci, 2013, 4(7): 2941 doi: 10.1039/c3sc51052b [42] Lu J H, Lian F, Guan L L, et al. Adapting FeS2 micron particles as an electrode material for lithium-ion batteries via simultaneous construction of CNT internal networks and external cages. J Mater Chem A, 2019, 7(3): 991 doi: 10.1039/C8TA09955C [43] Tao S, Huang W F, Xie H, et al. Formation of graphene-encapsulated CoS2 hybrid composites with hierarchical structures for high-performance lithium-ion batteries. RSC Adv, 2017, 7(63): 39427 [44] Yang W F, Wang J W, Ma W S, et al. Free-standing CuO nanoflake arrays coated Cu foam for advanced lithium ion battery anodes. J Power Sources, 2016, 333: 88 doi: 10.1016/j.jpowsour.2016.09.154 [45] Hua X, Liu Z, Fischer M G, et al. Lithiation thermodynamics and kinetics of the TiO2(B) nanoparticles. J Am Chem Soc, 2017, 139(38): 13330 doi: 10.1021/jacs.7b05228 [46] Xu X D, Cao R G, Jeong S, et al. Spindle-like mesoporous α-Fe2O3 anode material prepared from MOF template for high-rate lithium batteries. Nano Lett, 2012, 12(9): 4988 doi: 10.1021/nl302618s [47] Xia G L, Su J W, Li M S, et al. A MOF-derived self-template strategy toward cobalt phosphide electrodes with ultralong cycle life and high capacity. J Mater Chem A, 2017, 5(21): 10321 doi: 10.1039/C7TA02600E [48] Shao J, Wan Z M, Liu H M, et al. Metal organic frameworks-derived Co3O4 hollow dodecahedrons with controllable interiors as outstanding anodes for Li storage. J Mater Chem A, 2014, 2(31): 12194 doi: 10.1039/C4TA01966K [49] Zheng F C, He M N, Yang Y, et al. Nano electrochemical reactors of Fe2O3 nanoparticles embedded in shells of nitrogen-doped hollow carbon spheres as high-performance anodes for lithium-ion batteries. Nanoscale, 2015, 7(8): 3410 doi: 10.1039/C4NR06321J [50] Wang B X, Wang Z Q, Cui Y J, et al. Cr2O3@TiO2 yolk/shell octahedrons derived from a metal-organic framework for high-performance lithium-ion batteries. Microporous Mesoporous Mater, 2015, 203: 86 doi: 10.1016/j.micromeso.2014.10.026 [51] Tan Y L, Zhu K, Li D, et al. N-doped graphene/Fe-Fe3C nano-composite synthesized by a Fe-based metal organic framework and its anode performance in lithium ion batteries. Chem Eng J, 2014, 258: 93 doi: 10.1016/j.cej.2014.07.066 [52] Shiva K, Jayaramulu K, Rajendra H B, et al. In-situ stabilization of tin nanoparticles in porous carbon matrix derived from metal organic framework: high capacity and high rate capability anodes for lithium-ion batteries. Zeitschrift für anorganische und allgemeine Chemie, 2014, 640(6): 1115 doi: 10.1002/zaac.201300621 [53] Guo H, Li T, Chen W, et al. General design of hollow porous CoFe2O4 nanocubes from metal-organic frameworks with extraordinary lithium storage. Nanoscale, 2014, 6(24): 15168 doi: 10.1039/C4NR04422C [54] Huang G, Zhang F F, Zhang L L, et al. Hierarchical NiFe2O4/Fe2O3 nanotubes derived from metal organic frameworks for superior lithium ion battery anodes. J Mater Chem A, 2014, 2(21): 8048 doi: 10.1039/C4TA00200H [55] Zheng F C, Zhu D Q, Shi X H, et al. Metal-organic framework-derived porous Mn1.8Fe1.2O4 nanocubes with an interconnected channel structure as high-performance anodes for lithium ion batteries. J Mater Chem A, 2015, 3(6): 2815 doi: 10.1039/C4TA06150K [56] Xu W W, Xie Z Q, Wang Z, et al. Interwoven heterostructural Co3O4-carbon@FeOOH hollow polyhedrons with improved electrochemical performance. J Mater Chem A, 2016, 4(48): 19011 doi: 10.1039/C6TA08217C [57] Fang G Z, Wu Z X, Zhou J, et al. Observation of pseudocapacitive effect and fast ion diffusion in bimetallic sulfides as an advanced sodium-ion battery anode. Adv Energy Mater, 2018, 8(19): 1703155 doi: 10.1002/aenm.201703155 [58] Wang X, Chen Y, Fang Y J, et al. Synthesis of cobalt sulfide multi-shelled nanoboxes with precisely controlled two to five shells for sodium-ion batteries. Angew Chem Int Ed, 2019, 58(9): 2675 doi: 10.1002/anie.201812387 [59] Zou F, Chen Y M, Liu K W, et al. Metal organic frameworks derived hierarchical hollow NiO/Ni/graphene composites for lithium and sodium storage. ACS Nano, 2015, 10(1): 377 [60] Ding Y C, Hu L H, He D C, et al. Design of multishell microsphere of transition metal oxides/carbon composites for lithium ion battery. Chem Eng J, 2020, 380: 122489 doi: 10.1016/j.cej.2019.122489 [61] Hu H, Zhang J T, Guan B Y, et al. Unusual formation of CoSe@carbon nanoboxes, which have an inhomogeneous shell, for efficient lithium storage. Angew Chem, 2016, 128(33): 9666 doi: 10.1002/ange.201603852 [62] Wang J L, Wang J W, Han L F, et al. Fabrication of an anode composed of a N, S co-doped carbon nanotube hollow architecture with CoS2 confined within: toward Li and Na storage. Nanoscale, 2019, 11(43): 20996 doi: 10.1039/C9NR07767G [63] Zhang L, Wu H B, Lou X W. MOFs-derived general formation of hollow structures with high complexity. J Am Chem Soc, 2013, 135(29): 10664 doi: 10.1021/ja401727n [64] Zhang J T, Hu H, Li Z, et al. Double-shelled nanocages with cobalt hydroxide inner shell and layered double hydroxides outer shell as high-efficiency polysulfide mediator for lithium-sulfur batteries. Angew Chem Int Ed, 2016, 55(12): 3982 doi: 10.1002/anie.201511632 [65] Zhang L, Wu H B, Madhavi S, et al. Formation of Fe2O3 microboxes with hierarchical shell structures from metal-organic frameworks and their lithium storage properties. J Am Chem Soc, 2012, 134(42): 17388 doi: 10.1021/ja307475c [66] Yu L, Yang J F, Lou X W. Formation of CoS2 nanobubble hollow prisms for highly reversible lithium storage. Angew Chem Int Ed, 2016, 55(43): 13422 doi: 10.1002/anie.201606776 [67] Li P H, Yang Y, Gong S, et al. Co-doped 1T-MoS2 nanosheets embedded in N, S-doped carbon nanobowls for high-rate and ultra-stable sodium-ion batteries. Nano Res, 2019, 12(9): 2218 doi: 10.1007/s12274-018-2250-2 [68] Guo W X, Sun W W, Lü L P, et al. Microwave-assisted morphology evolution of Fe-based metal-organic frameworks and their derived Fe2O3 nanostructures for Li-ion storage. ACS Nano, 2017, 11(4): 4198 doi: 10.1021/acsnano.7b01152 [69] Wu R B, Wang D P, Rui X H, et al. In-situ formation of hollow hybrids composed of cobalt sulfides embedded within porous carbon polyhedra/carbon nanotubes for high-performance lithium-ion batteries. Adv Mater, 2015, 27(19): 3038 doi: 10.1002/adma.201500783 [70] Zhang H, Wang Y S, Zhao W Q, et al. MOF-derived ZnO nanoparticles covered by N-doped carbon layers and hybridized on carbon nanotubes for Lithium-ion battery anodes. ACS Appl Mater Interfaces, 2017, 9(43): 37813 doi: 10.1021/acsami.7b12095 [71] Xu X L, Wang H, Liu J B, et al. The applications of zeolitic imidazolate framework-8 in electrical energy storage devices: a review. J Mater Sci Mater Electron, 2017, 28: 7532 doi: 10.1007/s10854-017-6485-6 [72] Ghimbeu C M, Górka J, Simone V, et al. Insights on the Na+ ion storage mechanism in hard carbon: discrimination between the porosity, surface functional groups and defects. Nano Energy, 2018, 44: 327 doi: 10.1016/j.nanoen.2017.12.013 [73] Sathiya M, Rousse G, Ramesha K, et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat Mater, 2013, 12: 827 doi: 10.1038/nmat3699 [74] Nayak P K, Erickson E M, Schipper F, et al. Review on challenges and recent advances in the electrochemical performance of high capacity Li- and Mn- rich cathode materials for Li-ion batteries. Adv Energy Mater, 2018, 8(8): 1702397 doi: 10.1002/aenm.201702397 [75] Li W, Liu J, Zhao D Y. Mesoporous materials for energy conversion and storage devices. Nat Rev Mater, 2016, 1: 16023 doi: 10.1038/natrevmats.2016.23 -




 下載:
下載: