Development status and research progress of power battery for pure electric vehicles
-
摘要: 現階段, 鋰離子電池已經成為電動汽車最重要的動力源, 其發展經歷了三代技術的發展(鈷酸鋰正極為第一代, 錳酸鋰和磷酸鐵鋰為第二代, 三元技術為第三代).隨著正負極材料向著更高克容量的方向發展和安全性技術的日漸成熟、完善, 更高能量密度的電芯技術正在從實驗室走向產業化.本文從鋰離子電池產學研結合的角度, 從電池正負極材料, 電池設計和生產工藝來分析動力電池行業最新動態和科學研究的前沿成果, 并結合市場需求與政策導向來闡述動力電池的發展方向和技術路線的實現途徑.Abstract: Compared to the traditional electrochemical power source, lithium ion batteries (LIBs) have the advantages of higher energy density, longer life, and absence of any memory effect, and thus have attracted widespread research interest around the world. After Sony Inc. invented and produced the first commercial 18650 cell, many domestic and international research centers and companies have promoted the industrialization of LIBs. With the development of LIB technology, its application scope has extended from traditional consumer electronics to the new energy vehicles (NEVs) and energy storage fields. NEVs include pure electric vehicles (PEVs), hybrid electric vehicles (HEVs), and plug-in hybrid electric vehicles (PHEVs). LIBs have been the main driving force for PEVs to date, and their cathode technology development process has had three generations, i.e., the first using LiCoO2, the second using LiMn2O4 and LiFePO4, and the third generation using Li(NixCoyMn1-x-y)O2. With the development of cathode and anode materials with higher capacities and the increased reliability of LIB safety technology (including separators with higher temperature resistance, electrolytes with higher voltage resistance, and other protection methods), cells with higher energy densities and longer lives can be developed and applied in the future. These improvements will enable PEVs to travel longer distances, which is the most critical issue to customers. This paper provides a review of the development status of the power battery industry and an analysis of the direction of LIB technology with respect to the following: (1) the cathode/anode materials used, including the higher Ni content in Li(NixCoyMn1-x-y)O2, along with its structural modification, and the stability of silicon and improvements in its efficiency and cycle life; (2) the design technology, including the electrode and structure designs developed using simulation technology, theoretical modeling, and experimental methods based on Taguchi design; and (3) advances in process technology, including mixing and coating processes. Based on the above information, a clear picture of the technical direction was provided for LIBs in the PEV field.
-
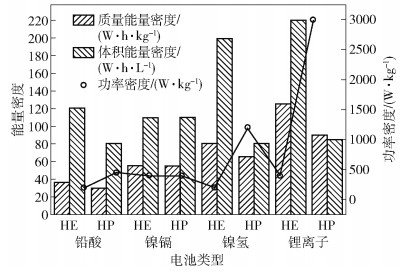
圖 1 不同類型電池的比功率/比能量曲線圖[1]
Figure 1. Ragone plot of different battery chemistries for electric vehicles
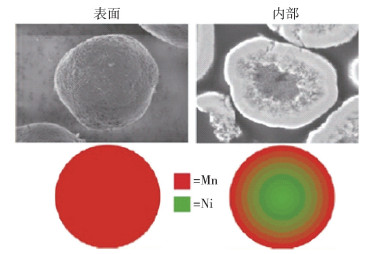
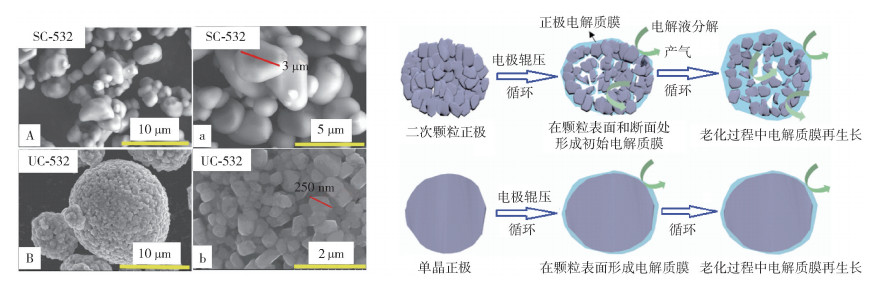
圖 11 單晶與多晶的三元材料顆粒的對比圖. (a) 單晶材料(A, a)與多晶材料(B, b)的掃描電鏡對比圖[30];(b) 電化學和界面上的穩定性對比的示意圖[32]
Figure 11. Comparison between polycrystalline and single crystalline NCM cathodes: (a) SEM morphologies of single-crystalline (A, a) and polycrystalline (B, b) NCM cathode[30]; (b) electrochemical and interfacial stability of polycrystalline and single-crystalline cathodes[32]
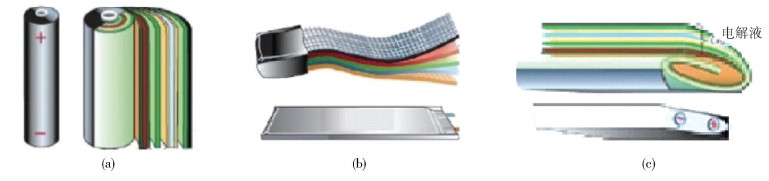
圖 17 動力電池的三種封裝形狀[56]. (a) 圓柱卷繞式;(b) 軟包疊片式;(c) 方形卷繞式
Figure 17. Three package structures of power batteries: (a) cylindrical type; (b) laminated type; (c) prismatic type
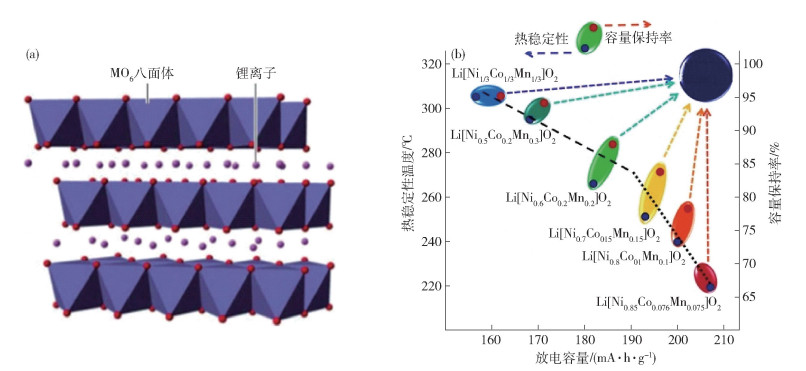
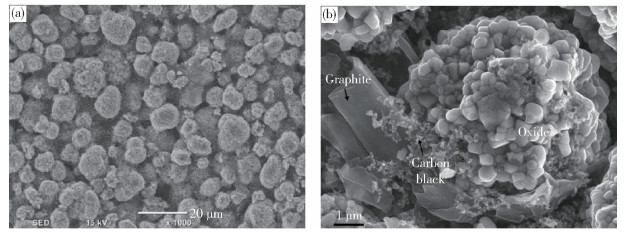
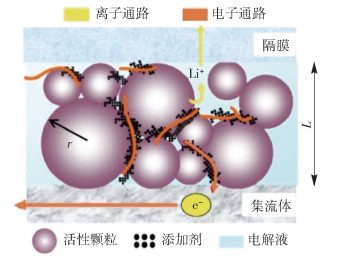
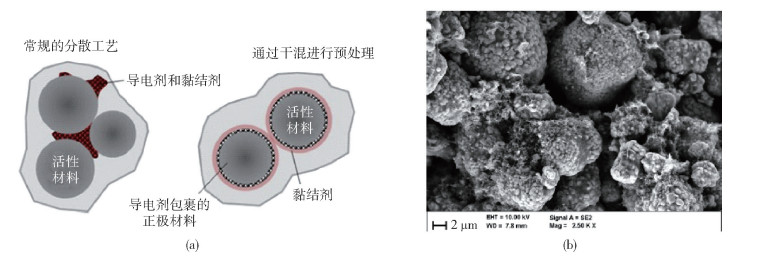
圖 20 活性顆粒表面導電網絡分布[67]. (a) 有無干法混料的導電劑分布示意圖;(b) 掃描電子顯微鏡下顆粒表面導電劑分布圖
Figure 20. Conductive network on the surface of active material particles[67]: (a) diagram of conductive agent coating on particles with and without dry mixing; (b) diagram of conductive agent coating on particles with dry mixing, characterized by SEM
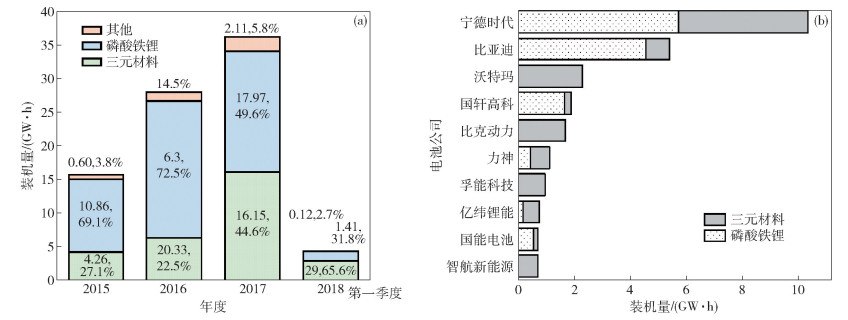
表 1 全球動力電池企業發展現狀(數據截止2017年)
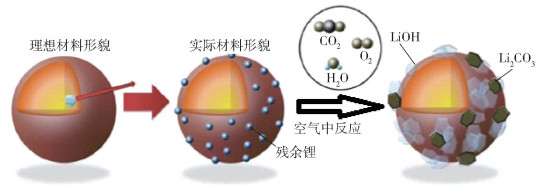
Table 1. Development status of global power battery enterprises (up to 2017)
銷量排名 動力電池企業 國家 電池銷量/(GW·h) 產品類型 能量密度/(W·h·kg-1) 主要客戶 1 寧德時代 中國 12 方型 200~250 寶馬,奔馳,大眾 2 松下 日本 10 圓柱 250~340 特斯拉 3 比亞迪 中國 7.2 方型 150~200 比亞迪 4 沃特瑪 中國 5.5 圓柱 145 奇瑞,金龍 5 LG化學 韓國 4.5 軟包 240 通用,現代,大眾 6 國軒高科 中國 3.2 方型 150~200 北汽,江鈴,長安 7 三星SDI 韓國 2.8 方型 250 寶馬 8 北京國能 中國 1.9 軟包 160~200 金龍,安凱 9 比克 中國 1.6 圓柱 220~232 眾泰,一汽,江淮 10 孚能科技 中國 1.3 軟包 220 北汽,長安 表 2 不同正極材料在電動汽車上的典型應用
Table 2. Application of positive materials in batteries for electric vehicles
汽車廠商 正極材料 電池供應商 電池總容量/(kW·h) 續航里程/km 特斯拉Model S NCA 松下 85.0 480 現代Kona NCM LG化學 64.0 470 寶馬i3 NCM 三星SDI 33.2 277 比亞迪e6 LFP 比亞迪 60.0 300 日產Leaf LMO+NCA AESC 24.0 160 表 3 目前商業化負極材料性能分析及應用情況
Table 3. Performance analysis and application of commercialized anode materials
材料 克容量/(mA·h·g-1) 首次效率/% 壓實密度/(g·m-3) 工作電壓/V 循環壽命(次) 安全性 倍率性能 應用方向 天然石墨 340~370 90~93 1.6~1.85 0.2 >1000 一般 差 數碼/動力 人造石墨 310~370 90~96 1.5~1.8 0.2 >1500 良好 良好 數碼/動力 中間相碳微球 280~340 90~94 1.5~1.7 0.2 >1000 良好 優秀 動力 軟碳 250~300 80~85 1.3~1.5 ~0.5 >1000 良好 優秀 動力/儲能 硬碳 250~400 80~85 1.3~1.5 ~0.5 >1000 良好 優秀 動力/儲能 鈦酸鋰 160~170 98~99 1.8~2.3 1.55 >30000 優秀 優秀 動力/儲能 硅/氧化硅 1000~4000 60~90 0.9~1.1 0.3~0.5 < 1000 一般 一般 動力 表 4 硅基材料制備工藝對比
Table 4. Comparison of various synthetic procedures for silicon-based materials
制備工藝 優勢 劣勢 高溫熱解法 1.工藝簡單,生產難度小
2.產品一致性較好1.顆粒不易分散,碳層包覆一致性不高
2.高溫處理過程顆粒易團聚,影響性能機械球磨法 1.可調控粒徑分布
2.生產成本較低1.需要根據硅與石墨的親和性選擇合適研磨條件
2.產生較多微晶顆粒,易引發副反應化學氣相沉積法 1.碳包覆層均勻性好
2.對材料性能提升明顯1.工藝設備投入復雜,成本高
2.需要與其他方法組合使用表 5 硅基材料對比
Table 5. Performance comparison of different silicon-based materials
硅基材料 優勢 劣勢 氧化硅 1、可逆容量高,體積膨脹低
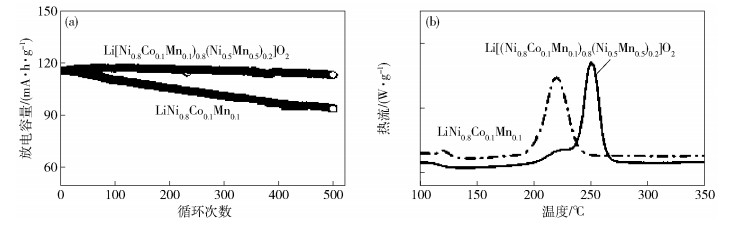
2、循環和倍率性能相比硅負極較好1、首次效率低(< 80%)
2、制備工藝復雜,成本較高硅 1、克容量發揮高,首次效率更高
2、工藝成熟,原材料便宜1、體積膨脹率大,循環性能差
2、熱安全性差硅合金 1、可逆容量高,體積膨脹低
2、體積和質量能量密度較低1、工藝制備復雜,成本高
2、首次效率和循環性能有待提高表 6 不同商業化的圓柱型動力電池型號及極耳設計方式
Table 6. Various tab layouts of cylindrical power batteries

表 7 高比能量電池設計思路
Table 7. Design concepts of high-energy cells
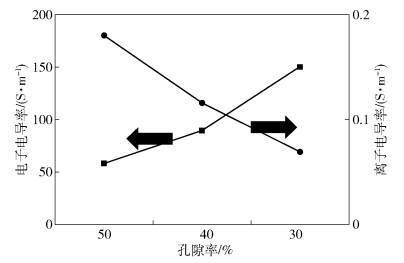
電池設計 途徑 實現方法 高克容量 高鎳三元材料+硅碳材料 材料配方 高主材占比 高導電性導電劑、高黏度黏結劑 微觀電極 高電壓 單晶材料、特殊電解液 面密度 提高料區面密度 提高料區涂覆量,降低箔材面密度 孔隙率 高壓實 單晶或粒徑分布合理范圍的三元材料 構型 極片組裝和封裝方式 軟包疊片式 宏觀結構 極片總面積 單位面積/電極數量 增加電極面積、卷繞圈數或疊片數 比例 箔材、隔膜和極耳 箔材和隔膜減薄,極耳結構優化 表 8 不同廠商的高比能量電池研究現狀
Table 8. Current research on high-energy cells by various battery manufacturers
廠商 構型 容量/(A·h) 能量密度/(W·h·kg-1) 體系 應用 松下 圓柱 4.1 242 NCA-硅碳 Model 3 比克 圓柱 2.9 220 811-石墨 云度π1、π3 CATL 方形 51 220 622-石墨 蔚來ES8 LG 軟包 56.7 212 532(4.3V)-石墨 通用 久色视频表 9 動力電池生產企業采用的涂布技術的優缺點
Table 9. Advantages and disadvantages of coatings adopted by mainstream LIB companies
涂布技術 優點 缺點 刮刀轉移式涂布 設備成本低
維護成本很低涂布速度慢
膜厚難以控制
涂布缺陷多
黏度范圍窄涂布速度高狹縫擠壓式涂布 膜厚一致性好
黏度范圍廣
涂布缺陷少
漿料利用率高設備成本高
安裝和操作要求高
模頭維護成本高 -
參考文獻
[1] An F Q, Qi L, Wang J, et al. Studies on power Li-ion secondary battery system for EV and HEV. Acta Sci Nat Univ Pek, 2011, 47(1): 1 https://www.cnki.com.cn/Article/CJFDTOTAL-BJDZ201101002.htm安富強, 其魯, 王劍, 等. 電動汽車用動力鋰離子二次電池系統性能的研究. 北京大學學報(自然科學版), 2011, 47(1): 1 https://www.cnki.com.cn/Article/CJFDTOTAL-BJDZ201101002.htm [2] Gallagher K G, Trask S E, Bauer C, et al. Optimizing areal capacities through understanding the limitations of lithium-ion electrodes. J Electrochem Soc, 2016, 163(2): A138 doi: 10.1149/2.0321602jes [3] Nitta N, Wu F X, Lee J T, et al. Li-ion battery materials: present and future. Mater Today, 2015, 18(5): 252 doi: 10.1016/j.mattod.2014.10.040 [4] Mizushima K, Jones P C, Wiseman P J, et al. LixCoO2 (0 < x < -1): a new cathode material for batteries of high energy density. Solid State Ionics, 1980, 15(6): 783 [5] Ohzuku T, Ueda A, Nagayama M. Electrochemistry and structural chemistry of LiNiO2 (R3m) for 4 volt secondary lithium cells. J Electrochem Soc, 1993, 140(7): 1862 doi: 10.1149/1.2220730 [6] Bruce P G, Robert Armstrong A, Gitzendanner R L. New intercalation compounds for lithium batteries: layered LiMnO2. J Mater Chem, 1999, 9(1): 193 doi: 10.1039/a803938k [7] Meng Y S, Arroyo-de Dompablo M E. Recent advances in first principles computational research of cathode materials for lithium-ion batteries. Acc Chem Res, 2013, 46(5): 1171 doi: 10.1021/ar2002396 [8] Liu Z L, Yu A S, Lee J Y. Synthesis and characterization of LiNi1-x-yCoxMnyO2 as the cathode materials of secondary lithium batteries. J Power Sources, 1999, 81-82: 416 doi: 10.1016/S0378-7753(99)00221-9 [9] Shao Y J, Huang B, Liu Q B, et al. Preparation and modification of Ni-Co-Mn ternary cathode materials. Prog Chem, 2018, 30(4): 410 https://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ201804007.htm邵奕嘉, 黃斌, 劉全兵, 等. 三元鎳鈷錳正極材料的制備及改性. 化學進展, 2018, 30(4): 410 https://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ201804007.htm [10] Noh H J, Youn S, Yoon C S, et al. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x=1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J Power Sources, 2013, 233: 121 [11] Cao Y, Yan C C, Wang Y F, et al. The technical route exploration of lithium ion battery with high safety and high energy density. Energy Stor Sci Technol, 2018, 7(3): 384 https://www.cnki.com.cn/Article/CJFDTOTAL-CNKX201803003.htm曹勇, 嚴長青, 王義飛, 等. 高安全高比能量動力鋰離子電池系統路線探索. 儲能科學與技術, 2018, 7(3): 384 https://www.cnki.com.cn/Article/CJFDTOTAL-CNKX201803003.htm [12] Liu L, Bao S S, He H, et al. Research progress of Ni-rich ternary cathode materials for lithium ion batteries. Electron Compon Mater, 2017, 36(12): 58 https://www.cnki.com.cn/Article/CJFDTOTAL-DZAL201712012.htm劉磊, 包珊珊, 何歡, 等. 鋰離子電池富鎳三元正極材料研究進展. 電子元件與材料, 2017, 36(12): 58 https://www.cnki.com.cn/Article/CJFDTOTAL-DZAL201712012.htm [13] Cho D H, Jo C H, Cho W, et al. Effect of residual lithium compounds on layer Ni-rich Li[Ni0.7Mn0.3]O2. J Electrochem Soc, 2014, 161(6): A920 doi: 10.1149/2.042406jes [14] Zhai Y W, Zhang J C, Zhao H, et al. Research progress of ternary layered oxide cathode materials for lithium ion batteries. J Eng Stud, 2017, 9(6): 523 https://www.cnki.com.cn/Article/CJFDTOTAL-GCKG201706001.htm翟彥武, 張繼成, 趙虎, 等. 鋰離子電池三元層狀氧化物正極材料的研究進展. 工程研究-跨學科視野中的工程, 2017, 9(6): 523 https://www.cnki.com.cn/Article/CJFDTOTAL-GCKG201706001.htm [15] Fu C Y, Zhou Z L, Liu Y H, et al. Synthesis and electrochemical properties of Mg-doped LiNi0.6Co0.2Mn0.2O2 cathode materials for Li-ion battery. J Wuhan Univ Technol-Mater Sci Ed, 2011, 26(2): 211 doi: 10.1007/s11595-011-0199-z [16] Ding Y H, Zhang P, Long Z L, et al. Morphology and electrochemical properties of Al doped LiNi1/3Co1/3Mn1/3O2 nanofibers prepared by electrospinning. J Alloys Compd, 2009, 487(1-2): 507 doi: 10.1016/j.jallcom.2009.08.002 [17] Kam K C, Mehta A, Heron J T, et al. Electrochemical and physical properties of Ti-substituted layered nickel manganese cobalt oxide (NMC) cathode materials. J Electrochem Soc, 2012, 159(8): A1383 doi: 10.1149/2.060208jes [18] Woo S U, Park B C, Yoon C S, et al. Improvement of electrochemical performances of LiNi0.8Co0.1Mn0.1O2 cathode materials by Fluorine substitution. J Electrochem Soc, 2007, 154(7): A649 doi: 10.1149/1.2735916 [19] Yan J, Yuan W, Tang Z Y, et al. Synthesis and electrochemical performance of Li3V2(P04)3-xClx/C cathode materials for lithium-ion batteries. J Power Sources, 2012, 209: 251 doi: 10.1016/j.jpowsour.2012.02.110 [20] Huang Y D, Jiang R R, Jia D Z, et al. Preparation, microstructure and electrochemical performance of nanoparticles LiMn2O3.9Br0.1. Mater Lett, 2011, 65(23-24): 3486 doi: 10.1016/j.matlet.2011.07.091 [21] Woo S W, Myung S T, Bang H, et al. Improvement of electrochemical and thermal properties of Li[Ni0.8Co0.1Mn0.1]O2 positive electrode materials by multiple metal (Al, Mg) substitution. Electrochim Acta, 2009, 54(15): 3851 doi: 10.1016/j.electacta.2009.01.048 [22] Shin H S, Shin D, Sun Y K. Improvement of electrochemical properties of Li[Ni0.4Co0.2Mn(0.4-x)Mgx]O2-yFy cathode materials at high voltage region. Electrochim Acta, 2006, 52(4): 1477 doi: 10.1016/j.electacta.2006.02.048 [23] Dong M X, Wang Z X, Li H K, et al. Metallurgy inspired formation of homogeneous Al2O3 coating layer to improve the electrochemical properties of LiNi0.8Co0.1Mn0.1O2 Cathode Material. ACS Sustainable Chem Eng, 2017, 5(11): 10199 doi: 10.1021/acssuschemeng.7b02178 [24] Cho J, Kim T J, Kim Y J, et al. High-performance ZrO2-coated LiNiO2 cathode material. Electrochem Solid-State Lett, 2001, 4(10): A159 doi: 10.1149/1.1398556 [25] Kweon H J, Kim S J, Park D G. Modification of LixNi1·yCoyO2 by applying a surface coating of MgO. J Power Sources, 2000, 88(2): 255 doi: 10.1016/S0378-7753(00)00368-2 [26] Ying J R, Wan C R, Jiang C Y. Surface treatment of LiNi0.8Co0.2O2 cathode material for lithium secondary batteries. J Power Sources, 2001, 102(1-2): 162 doi: 10.1016/S0378-7753(01)00795-9 [27] Zhang J C, Zhang H, Gao R, et al. New insights into the modification mechanism of Li-rich Li1.2Mn0.6Ni0.2O2 coated by Li2ZrO3. Phys Chem Chem Phys, 2016, 18(19): 13322 doi: 10.1039/C6CP01366J [28] Liu S J, Wu H, Huang L, et al. Synthesis of Li2Si2O5-coated LiNi0.6Co0.2Mn0.2O2 cathode materials with enhanced high-voltage electrochemical properties for lithium-ion batteries. J Alloys Compd, 2016, 674: 447 doi: 10.1016/j.jallcom.2016.03.060 [29] Sun Y K, Myung S T, Kim M H, et al. Synthesis and characterization of Li[(Ni0.8Co0.1Mn0.1)0.8(Ni0.5Mn0.5)0.2]O2 with the microscale core-shell structure as the positive electrode material for lithium batteries. J Am Chem Soc, 2005, 127(38): 13411 doi: 10.1021/ja053675g [30] Koenig Jr G M, Belharouak I, Deng H X, et al. Composition-tailored synthesis of gradient transition metal precursor particles for lithium-ion battery cathode materials. Chem Mater, 2011, 23(7): 1954 doi: 10.1021/cm200058c [31] Xiao J W, Liu L B, Fu Z W, et al. Research progress in the single crystal LiNixCoyMn1-x-yO2 ternary cathode materials. Chin Battery Ind, 2017, 21(2): 51 doi: 10.3969/j.issn.1008-7923.2017.02.013肖建偉, 劉良彬, 符澤衛, 等. 單晶LiNixCOyMn1-x-yO2三元正極材料研究進展. 電池工業, 2017, 21(2): 51 doi: 10.3969/j.issn.1008-7923.2017.02.013 [32] Li J, Cameron A R, Li H Y, et al. Comparison of single crystal and polycrystalline LiNi0.5Mn0.3Co0.2O2 positive electrode materials for high voltage Li-ion cells. J Electrochem Soc, 2017, 164(7): A1534 doi: 10.1149/2.0991707jes [33] Kim J, Lee H, Cha H, et al. Prospect and reality of Ni-rich cathode for commercialization. Adv Energy Mater, 2017, 8(6): 1702028 doi: 10.1002/aenm.201702028 [34] Kim Y. Lithium nickel cobalt manganese oxide synthesized using alkali chloride flux: morphology and performance as a cathode material for lithium ion batteries. ACS Appl Mater Interfaces, 2012, 4(5): 2329 doi: 10.1021/am300386j [35] Sun Y M, Zheng G Y, Seh Z W, et al. Graphite-encapsulated Li-metal hybrid anodes for high-capacity Li batteries. Chem, 2016, 1(2): 287 doi: 10.1016/j.chempr.2016.07.009 [36] Dash R, Pannala S. Theoretical limits of energy density in silicon-carbon composite anode based lithium ion batteries. Sci Rep, 2016, 6: 27449 doi: 10.1038/srep27449 [37] Jo Y N, Kim Y, Kim J S, et al. Si-graphite composites as anode materials for lithium secondary batteries. J Power Sources, 2010, 195(18): 6031 doi: 10.1016/j.jpowsour.2010.03.008 [38] Lee J H, Kim W J, Kim J Y, et al. Spherical silicon/graphite/carbon composites as anode material for lithium-ion batteries. J Power Sources, 2008, 176(1): 353 doi: 10.1016/j.jpowsour.2007.09.119 [39] Ma C L, Ma C, Wang J Z, et al. Exfoliated graphite as a flexible and conductive support for Si-based Li-ion battery anodes. Carbon, 2014, 72: 38 doi: 10.1016/j.carbon.2014.01.027 [40] Sun Q, Zhang B, Fu Z W. Lithium electrochemistry of SiO2 thin film electrode for lithium-ion batteries. Appl Surf Sci, 2008, 254(13): 3774 doi: 10.1016/j.apsusc.2007.11.058 [41] Philippe B, Dedryvère R, Allouche J, et al. Nanosilicon electrodes for lithium-ion batteries: interfacial mechanisms studied by hard and soft X-ray photoelectron spectroscopy. Chem Mater, 2012, 24(6): 1107 doi: 10.1021/cm2034195 [42] Kim H J, Choi S, Lee S J, et al. Controlled prelithiation of silicon monoxide for high performance lithium-ion rechargeable full cells. Nano Lett, 2016, 16(1): 282 doi: 10.1021/acs.nanolett.5b03776 [43] Li X M, Kersey-Bronec F E, Ke J, et al. Study of lithium silicide nanoparticles as anode materials for advanced lithium ion batteries. ACS Appl Mater Interfaces, 2017, 9(19): 16071 doi: 10.1021/acsami.6b16773 [44] Zhao J, Lu Z D, Wang H T, et al. Artificial solid electrolyte interphase-protected LixSi nanoparticles: an efficient and stable prelithiation reagent for lithium-ion batteries. J Am Chem Soc, 2015, 137(26): 8372 doi: 10.1021/jacs.5b04526 [45] Zhao H, Wang Z H, Lu P, et al. Toward practical application of functional conductive polymer binder for a high-energy lithium-ion battery design. Nano Lett, 2014, 14(11): 6704 doi: 10.1021/nl503490h [46] Su H P, Barragan A A, Geng L X, et al. Colloidal synthesis of silicon-carbon composite materials for lithium-ion batteries. Angew Chem, 2017, 129(36): 10920 doi: 10.1002/ange.201705200 [47] Palomino J, Varshney D, Weiner B R, et al. Study of the structural changes undergone by hybrid nanostructured Si-CNTs employed as an anode material in a rechargeable lithium-ion battery. J Phys Chem C, 2015, 119(36): 21125 doi: 10.1021/acs.jpcc.5b01178 [48] Wang Y D, Jiang J W, Dahn J R. The reactivity of delithiated Li(Ni1/3Co1/3Mn1/3)O2, Li(Ni0.8Co0.15Al0.05)O2 or LiCoO2 with non-aqueous electrolyte. Electrochem Commun, 2007, 9(10): 2534 doi: 10.1016/j.elecom.2007.07.033 [49] Wang Z H. Modeling of Porous Electrode Using Stacked-Agglomerates[Dissertation]. Beijing: Tsinghua University, 2017王子珩. 團聚體堆疊型多孔電極模型構建與應用[學位論文]. 北京: 清華大學, 2017 [50] Chen Y H, Wang C W, Zhang X, et al. Porous cathode optimization for lithium cells: ionic and electronic conductivity, capacity, and selection of materials. J Power Sources, 2010, 195(9): 2851 doi: 10.1016/j.jpowsour.2009.11.044 [51] Yu S, Chung Y, Song M S, et al. Investigation of design parameter effects on high current performance of lithium-ion cells with LiFePO4/graphite electrodes. J Appl Electrochem, 2012, 42(6): 443 doi: 10.1007/s10800-012-0418-0 [52] Appiah W A, Park J, Song S, et al. Design optimization of LiNi0.6Co0.2Mn0.2O2/graphite lithium-ion cells based on simulation and experimental data. J Power Sources, 2016, 319: 147 doi: 10.1016/j.jpowsour.2016.04.052 [53] De S, Northrop P W C, Ramadesigan V, et al. Model-based simultaneous optimization of multiple design parameters for lithium-ion batteries for maximization of energy density. J Power Sources, 2013, 227: 161 doi: 10.1016/j.jpowsour.2012.11.035 [54] Xue N S, Du W B, Gupta A, et al. Optimization of a single lithium-ion battery cell with a gradient-based algorithm. J Electrochem Soc, 2013, 160(8): A1071 doi: 10.1149/2.036308jes [55] Golmon S, Maute K, Dunn M L. Multiscale design optimization of lithium ion batteries using adjoint sensitivity analysis. Int J Numer Methods Eng, 2012, 92(5): 475 doi: 10.1002/nme.4347 [56] Meng F W. Studies on the Design and Performance of Lithium Ion Battery[Dissertation]. Beijing: Beijing Institute of Technology, 2015孟凡偉. 鋰離子電池的相關設計與性能研究[學位論文]. 北京: 北京理工大學, 2015 [57] Li P, An F Q, Zhang J B, et al. Temperature sensitivity of lithium-ion battery: a review. J Autom Saf Energy, 2014, 5(3): 224 doi: 10.3969/j.issn.1674-8484.2014.03.002李平, 安富強, 張劍波, 等. 電動汽車用鋰離子電池的溫度敏感性研究綜述. 汽車安全與節能學報, 2014, 5(3): 224 doi: 10.3969/j.issn.1674-8484.2014.03.002 [58] Zhang J B, Li Z, Wu B. The Structure Design Theory and Application of Lithium-Ion Battery. Beijing: Science and Technology of China Press, 2016張劍波, 李哲, 吳彬. 鋰離子電池結構設計理論與應用. 北京: 中國科學技術出版社, 2016 [59] Al-Hallaj S, Selman J R. Thermal modeling of secondary lithium batteries for electric vehicle/hybrid electric vehicle applications. J Power Sources, 2002, 110(2): 341 doi: 10.1016/S0378-7753(02)00196-9 [60] Chen S C, Wang Y Y, Wan C C. Thermal analysis of spirally wound lithium batteries. J Electrochem Soc, 2006, 153(4): A637 doi: 10.1149/1.2168051 [61] Kim U S, Shin C B, Kim C S. Effect of electrode configuration on the thermal behavior of a lithium-polymer battery. J Power Sources, 2008, 180(2): 909 doi: 10.1016/j.jpowsour.2007.09.054 [62] Wu B. Thermal Design Methodology for Traction Lithium-Ion Batteries [Dissertation]. Beijing: Tsinghua University, 2015吳彬. 鋰離子動力電池熱設計方法研究[學位論文]. 北京: 清華大學, 2015 [63] Inui Y, Kobayashi Y, Watanabe Y, et al. Simulation of temperature distribution in cylindrical and prismatic lithium ion secondary batteries. Energy Convers Manage, 2007, 48(7): 2103 doi: 10.1016/j.enconman.2006.12.012 [64] Zhao W, Luo G, Wang C Y. Effect of tab design on large-format Li-ion cell performance. J Power Sources, 2014, 257: 70 doi: 10.1016/j.jpowsour.2013.12.146 [65] Lee K J, Smith K, Pesaran A, et al. Three dimensional thermal-, electrical-, and electrochemical-coupled model for cylindrical wound large format lithium-ion batteries. J Power Sources, 2013, 241: 20 doi: 10.1016/j.jpowsour.2013.03.007 [66] Kim K M, Jeon W S, Chung I J, et al. Effect of mixing sequences on the electrode characteristics of lithium-ion rechargeable batteries. J Power Sources, 1999, 83(1-2): 108 doi: 10.1016/S0378-7753(99)00281-5 [67] Bauer W, N?tzel D, Wenzel V, et al. Influence of dry mixing and distribution of conductive additives in cathodes for lithium ion batteries. J Power Sources, 2015, 288: 359 doi: 10.1016/j.jpowsour.2015.04.081 [68] Bockholt H, Haselrieder W, Kwade A. Intensive powder mixing for dry dispersing of carbon black and its relevance for lithium-ion battery cathodes. PowderTechnol, 2016, 297: 266 http://www.sciencedirect.com/science/article/pii/S003259101630170X [69] Qian L, Zhu D, Rao M M, et al. The study of Li-ion battery cathode dispersion technology. Battery Bimonthly, 2016, 46(2): 95 doi: 10.3969/j.issn.1001-1579.2016.02.010錢龍, 朱丹, 饒睦敏, 等. 鋰離子電池負極分散工藝研究. 電池, 2016, 46(2): 95 doi: 10.3969/j.issn.1001-1579.2016.02.010 [70] Westphal B G, Mainusch N, Meyer C, et al. Influence of high intensive dry mixing and calendering on relative electrode resistivity determinedvia an advanced two point approach. J Energy Storage, 2017, 11: 76 doi: 10.1016/j.est.2017.02.001 [71] Jin G L, Ahn W G, Kim S J, et al. Effect of shim configuration on internal die flows for non-Newtonian coating liquids in slot coating process. Korea-Australia Rheology J, 2016, 28(2): 159 doi: 10.1007/s13367-016-0015-6 -




 下載:
下載: